Difference between revisions of "Polyurethane"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (57 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:1989.818-SC16408.jpg|thumb|]] | + | [[File:1989.818-SC16408.jpg|thumb|L'Enquêteur<br>MFA# 1989.818]] |
| + | [[File:Polyurethane chair.jpg|thumb|20x|Polyurethane chair<br>MFA# 1996.191]] | ||
== Description == | == Description == | ||
| − | A family of polymers made by a condensation reaction of an organic isocyanate with a compound containing a hydroxyl group, such as glycol. Polymers of this type (ester type) were first made in 1937 by Otto Bayer at I.G.Farben. During W.W.II, Germany made brush [[bristle|bristles]] and filtration fabrics from Perlon U, an early polyurethane. In the | + | A family of polymers made by a condensation reaction of an organic isocyanate with a compound containing a hydroxyl group, such as glycol. Polymers of this type ('''ester type''') were first made in 1937 by Otto Bayer at I.G.Farben. During W.W.II, Germany made brush [[bristle|bristles]] and filtration fabrics from Perlon U, an early polyurethane. In the 1950s another type of polyurethane using an ether starting compound ('''ether type''') was used to produce elastomeric polyurethane fiber called [[Spandex fiber|spandex]] or elastane. Spandex has elastic characteristics similar to [[natural rubber]]. Since the 1980s, water-blown flexible polyurethane foams were made for seals and gaskets, In the 90s, due to the elimination of halogen blowing agents, polyurethane foaming techniques were expanded to insulation materials, cushions and packaging. As solvent based liquids and solids, polyurethanes are used as [[sealant |sealants]], [[adhesive| adhesives]], [[film| films]], shopping carts, and automobile bumpers. Polyurethanes can be rigid or soft, [[thermoset| Thermosetting]] or [[thermoplastic]]. Additionally, they react with isocyanates to produce a foamed resin. Polyurethane resins are also used as [[coating |coatings]] where they provide excellent [[hardness (solids)|hardness]], [[gloss]], and resistance to [[weathering]], [[abrasion]], [[acid|acids]], and [[alkali |alkalis]]. |
| + | [[File:Ester-ether.jpg|thumb|Polyurethane foams; Image from: [https://www.paccin.org/content.php?278-Polyurethane-Foams PACCIN]]] | ||
| + | {| class="wikitable" | ||
| + | |+Table of Polyurethane Foams and their Characteristics | ||
| + | |- | ||
| + | ! Types!!Properties!!Forms!!Characteristics!!Uses | ||
| + | |- | ||
| + | |Poly'''ether''' urethane|| thermoset || flexible open cells || lightweight, resistant to water, allows vapor penetration, larger bubbles, slightly more flexible, less expensive, less stable, poor cushioning||speaker foam, aquarium filters, patio cushions, foam padding, mattresses, flotation devices, Dryfast foam | ||
| + | |- | ||
| + | |Poly'''ester''' urethane|| thermoset || flexible open cells || lightweight, resistant to solvents, allows vapor penetration, smaller bubbles, slightly more rigid, effective cushioning, better stability||packing foam, insulation, soundproofing, shock absorption, pink anti-static foam, mops, sponges | ||
| + | |- | ||
| + | |Poly'''ether''' urethane|| thermoset || rigid closed cells || dense, resistant to water, less expensive|| thermal and moisture insulation, vapor barrier, tool making, wood replacement | ||
| + | |- | ||
| + | |Poly'''ester''' urethane|| thermoset || rigid closed cells || dense, resistant to solvents||thermal, vapor barrier, tool making, wood replacement | ||
| + | |- | ||
| + | |Poly'''ether''' urethane|| thermoplastic (TPU) || soft to rigid || good resistance to abrasion, low temperature, moisture, and microbes||textiles, upholstery, elastane, wire coverings, hoses, films, laminates | ||
| + | |- | ||
| + | |Poly'''ester''' urethane|| thermoplastic (TPU) || soft to rigid ||good resistance to abrasion, solvents, light, and temperature|| skate wheels, safety helmets, power tools, medical devices, films, hoses, laminates, coatings | ||
| + | |} | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | PUR; poliuretano (Esp.); polyuréthane (Fr.); poliuretano (It.); poliuretano (Port.); spandex; elastane | + | PUR; PU: poliuretano (Esp.); polyuréthane (Fr.); poliuretano (It.); poliuretano (Port.); spandex; elastane |
| + | |||
| + | Examples: Perlon® U [Ger.]; [[Lycra|Lycra®]] [DuPont]; | ||
| − | + | == Personal Risks == | |
| + | * Urethane burns with a bright flame producing a sharp odor and toxic fumes. | ||
| + | * Polymer Plastics: [[https://www.polymerplastics.com/images/msds_sheets/Polyurethane.pdf Safety Data Sheet]] | ||
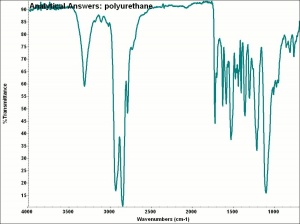
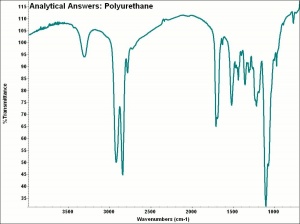
[[[SliderGallery rightalign|aaiCARDIO.jpg~FTIR|aaiPU-85.jpg~FTIR]]] | [[[SliderGallery rightalign|aaiCARDIO.jpg~FTIR|aaiPU-85.jpg~FTIR]]] | ||
| − | == | + | == Collection Risks == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | Polyether type urethanes are very susceptible to light degradation. | + | * Potential degradation products are hydrogen cyanide and ammonia. |
| + | * Polyether type urethanes are very susceptible to light degradation. | ||
| + | * Polyester type urethanes are very susceptible to degradation at high humidities. | ||
| + | * Polyurethane foams can yellow, become brittle and crumble. | ||
| + | * Chlorine bleach may cause degradation. | ||
| + | * Hydrolysis of polyester urethane foams can lead to loss of the structural support given by this material. Oxidation of polyether urethane foams is the primary deterioration method. (Lattuati-Derieux, 2011) | ||
| − | + | '''Links to Oddy Test results posted on AIC Wiki Materials Database Pages for individual materials below''' | |
| − | + | *[http://www.conservation-wiki.com/wiki/Oddy_Test_Results:_Exhibition_Adhesives_and_Tapes#Polyurethane0001 water-based Polyurethane] tested in 2003 | |
| − | == | + | == Physical and Chemical Properties == |
| − | + | * Coatings are resistant to weathering, abrasion, acids and alkalis. | |
| + | * Attacked by aromatic solvents, chlorinated solvents, ozone, and nitrogen oxides. | ||
| + | * Spot test for detection: dimethyl amino benzaldehyde in glacial acetic acid - positive reaction gives bright yellow color (Roff et al 1971) | ||
== Comparisons == | == Comparisons == | ||
| Line 46: | Line 58: | ||
[[media:download_file_87.pdf|Properties of Synthetic Fibers]] | [[media:download_file_87.pdf|Properties of Synthetic Fibers]] | ||
| − | [[media:|Physical Properties for Selected Thermoplastic Resins]] | + | [[media:download_file_363.pdf|Physical Properties for Selected Thermoplastic Resins]] |
[[media:download_file_296.pdf|General Characteristics of Polymers]] | [[media:download_file_296.pdf|General Characteristics of Polymers]] | ||
| − | + | == Resources and Citations == | |
| − | + | * PACCIN: [https://www.paccin.org/content.php?278-Polyurethane-Foams Polyurethane foams]]; ; [https://www.paccin.org/content.php?279-Polyurethane-Ester Polyurethane ester Foam]; [ https://www.paccin.org/content.php?277-Polyurethane-Ether Polyurethane ether Foam] Accessed Nov. 2023 | |
| − | == | + | * Lattuati-Derieux, A.; Thao-Heu, S.; Lavédrine, B. “Assessment of the degradation of polyurethane foams after artificial and natural ageing by using pyrolysis-gas chromatography/mass spectrometry and headspace-solid phase microextraction-gas chromatography/mass spectrometry” Journal of Chromatography A, 1218(28) 2011, 4498-4508. https://doi.org/10.1016/j.chroma.2011.05.013 |
| − | + | * Contributions: Molly McGath and Thea van Oossten, AIC Plastics Panel, 2020. | |
| − | * | + | * The Foam Factory at https://www.thefoamfactory.com/blog/index.php/ether-and-ester-based-polyurethane-foam-characteristics-differences-and-uses (Accessed July 2020) |
| − | + | * W.J.Roff, J.R.Scott, J.Pacitti (compilers) ''Handbook of Common Polymers:Fibres, Gilms, Plastics and Rubber'' Cleveland: CRC Press, Butterworth & Co., 1971. | |
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Polyurethane." | + | * C&E News Aug 2004 - first developed by Otto Bayer in 1937. |
| − | + | * Coughlin, Mary. “Plastics” Preventive Conservation: Collection Storage, edited by Lisa Elkin and Christopher A. Society for the Preservation of Natural History Collections, American Institute for Conservation of Historic and Artistic Works, Smithsonian Institution, The George Washington University Museum Studies Program, 2019. pp. 884-885. [https://spnhc.biowikifarm.net/w/media/c/c8/Ch53_Plastics_884-885_%282%29.pdf] | |
| + | * Williams, Scott R. “Plastic Storage Products” Preventive Conservation: Collection Storage, edited by Lisa Elkin and Christopher A. Society for the Preservation of Natural History Collections, American Institute for Conservation of Historic and Artistic Works, Smithsonian Institution, The George Washington University Museum Studies Program, 2019, pp. 753-780. [https://spnhc.biowikifarm.net/wiki/Storage_Materials:_Plastics] | ||
| + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Polyurethane." 18 Aug. 2004 | ||
* Marjorie Shelley, ''The Care and Handling of Art Objects'', The Metropolitan Museum, New York, 1987 | * Marjorie Shelley, ''The Care and Handling of Art Objects'', The Metropolitan Museum, New York, 1987 | ||
| − | |||
* Thomas C. Jester (ed.), ''Twentieth-Century Building Materials'', McGraw-Hill Companies, Washington DC, 1995 | * Thomas C. Jester (ed.), ''Twentieth-Century Building Materials'', McGraw-Hill Companies, Washington DC, 1995 | ||
| − | |||
* Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | ||
| − | |||
* ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | * ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | ||
| − | |||
* Sharon Blank, An introduction to plastics and rubbers in collections, ''Studies in Conservation'', 35, 53-63, 1990 | * Sharon Blank, An introduction to plastics and rubbers in collections, ''Studies in Conservation'', 35, 53-63, 1990 | ||
| − | |||
* M. Baker, E. McManus, 'History, Care and Handling of America's Spacesuits', ''JAIC'', 31, 77-85, 1992 | * M. Baker, E. McManus, 'History, Care and Handling of America's Spacesuits', ''JAIC'', 31, 77-85, 1992 | ||
| − | |||
* Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 | * Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 | ||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| − | |||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | * | + | * History of Plastics: www.me.umist.ac.uk.historyp/ |
| − | |||
| − | |||
| + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 14:33, 10 November 2023
Description
A family of polymers made by a condensation reaction of an organic isocyanate with a compound containing a hydroxyl group, such as glycol. Polymers of this type (ester type) were first made in 1937 by Otto Bayer at I.G.Farben. During W.W.II, Germany made brush bristles and filtration fabrics from Perlon U, an early polyurethane. In the 1950s another type of polyurethane using an ether starting compound (ether type) was used to produce elastomeric polyurethane fiber called spandex or elastane. Spandex has elastic characteristics similar to Natural rubber. Since the 1980s, water-blown flexible polyurethane foams were made for seals and gaskets, In the 90s, due to the elimination of halogen blowing agents, polyurethane foaming techniques were expanded to insulation materials, cushions and packaging. As solvent based liquids and solids, polyurethanes are used as sealants, adhesives, films, shopping carts, and automobile bumpers. Polyurethanes can be rigid or soft, Thermosetting or Thermoplastic. Additionally, they react with isocyanates to produce a foamed resin. Polyurethane resins are also used as coatings where they provide excellent hardness, Gloss, and resistance to Weathering, Abrasion, acids, and alkalis.

| Types | Properties | Forms | Characteristics | Uses |
|---|---|---|---|---|
| Polyether urethane | thermoset | flexible open cells | lightweight, resistant to water, allows vapor penetration, larger bubbles, slightly more flexible, less expensive, less stable, poor cushioning | speaker foam, aquarium filters, patio cushions, foam padding, mattresses, flotation devices, Dryfast foam |
| Polyester urethane | thermoset | flexible open cells | lightweight, resistant to solvents, allows vapor penetration, smaller bubbles, slightly more rigid, effective cushioning, better stability | packing foam, insulation, soundproofing, shock absorption, pink anti-static foam, mops, sponges |
| Polyether urethane | thermoset | rigid closed cells | dense, resistant to water, less expensive | thermal and moisture insulation, vapor barrier, tool making, wood replacement |
| Polyester urethane | thermoset | rigid closed cells | dense, resistant to solvents | thermal, vapor barrier, tool making, wood replacement |
| Polyether urethane | thermoplastic (TPU) | soft to rigid | good resistance to abrasion, low temperature, moisture, and microbes | textiles, upholstery, elastane, wire coverings, hoses, films, laminates |
| Polyester urethane | thermoplastic (TPU) | soft to rigid | good resistance to abrasion, solvents, light, and temperature | skate wheels, safety helmets, power tools, medical devices, films, hoses, laminates, coatings |
Synonyms and Related Terms
PUR; PU: poliuretano (Esp.); polyuréthane (Fr.); poliuretano (It.); poliuretano (Port.); spandex; elastane
Examples: Perlon® U [Ger.]; Lycra® [DuPont];
Personal Risks
- Urethane burns with a bright flame producing a sharp odor and toxic fumes.
- Polymer Plastics: [Safety Data Sheet]
Collection Risks
- Potential degradation products are hydrogen cyanide and ammonia.
- Polyether type urethanes are very susceptible to light degradation.
- Polyester type urethanes are very susceptible to degradation at high humidities.
- Polyurethane foams can yellow, become brittle and crumble.
- Chlorine bleach may cause degradation.
- Hydrolysis of polyester urethane foams can lead to loss of the structural support given by this material. Oxidation of polyether urethane foams is the primary deterioration method. (Lattuati-Derieux, 2011)
Links to Oddy Test results posted on AIC Wiki Materials Database Pages for individual materials below
- water-based Polyurethane tested in 2003
Physical and Chemical Properties
- Coatings are resistant to weathering, abrasion, acids and alkalis.
- Attacked by aromatic solvents, chlorinated solvents, ozone, and nitrogen oxides.
- Spot test for detection: dimethyl amino benzaldehyde in glacial acetic acid - positive reaction gives bright yellow color (Roff et al 1971)
Comparisons
Properties of Synthetic Fibers
Physical Properties for Selected Thermoplastic Resins
General Characteristics of Polymers
Resources and Citations
- PACCIN: Polyurethane foams]; ; Polyurethane ester Foam; [ https://www.paccin.org/content.php?277-Polyurethane-Ether Polyurethane ether Foam] Accessed Nov. 2023
- Lattuati-Derieux, A.; Thao-Heu, S.; Lavédrine, B. “Assessment of the degradation of polyurethane foams after artificial and natural ageing by using pyrolysis-gas chromatography/mass spectrometry and headspace-solid phase microextraction-gas chromatography/mass spectrometry” Journal of Chromatography A, 1218(28) 2011, 4498-4508. https://doi.org/10.1016/j.chroma.2011.05.013
- Contributions: Molly McGath and Thea van Oossten, AIC Plastics Panel, 2020.
- The Foam Factory at https://www.thefoamfactory.com/blog/index.php/ether-and-ester-based-polyurethane-foam-characteristics-differences-and-uses (Accessed July 2020)
- W.J.Roff, J.R.Scott, J.Pacitti (compilers) Handbook of Common Polymers:Fibres, Gilms, Plastics and Rubber Cleveland: CRC Press, Butterworth & Co., 1971.
- C&E News Aug 2004 - first developed by Otto Bayer in 1937.
- Coughlin, Mary. “Plastics” Preventive Conservation: Collection Storage, edited by Lisa Elkin and Christopher A. Society for the Preservation of Natural History Collections, American Institute for Conservation of Historic and Artistic Works, Smithsonian Institution, The George Washington University Museum Studies Program, 2019. pp. 884-885. [1]
- Williams, Scott R. “Plastic Storage Products” Preventive Conservation: Collection Storage, edited by Lisa Elkin and Christopher A. Society for the Preservation of Natural History Collections, American Institute for Conservation of Historic and Artistic Works, Smithsonian Institution, The George Washington University Museum Studies Program, 2019, pp. 753-780. [2]
- Encyclopedia Britannica, http://www.britannica.com Comment: "Polyurethane." 18 Aug. 2004
- Marjorie Shelley, The Care and Handling of Art Objects, The Metropolitan Museum, New York, 1987
- Thomas C. Jester (ed.), Twentieth-Century Building Materials, McGraw-Hill Companies, Washington DC, 1995
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Sharon Blank, An introduction to plastics and rubbers in collections, Studies in Conservation, 35, 53-63, 1990
- M. Baker, E. McManus, 'History, Care and Handling of America's Spacesuits', JAIC, 31, 77-85, 1992
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- History of Plastics: www.me.umist.ac.uk.historyp/
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000