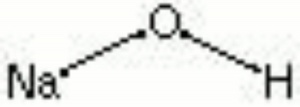Difference between revisions of "Sodium hydroxide"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | White, deliquescent solid. Sodium hydroxide was the 8th highest-volume chemical produced in the U.S. in 1991. It is primarily used in the manufacture of chemicals, paper, soaps ([ | + | White, deliquescent solid. Sodium hydroxide was the 8th highest-volume chemical produced in the U.S. in 1991. It is primarily used in the manufacture of chemicals, paper, soaps ([[castile%20soap|castile soap]]), cleaners (Drano) as well as for [[mercerized%20cotton|mercerizing cotton]], and neutralizing acids. It is also used to digest cellulose in making [[viscose%20rayon|viscose rayon]] and [[cellophane|cellophane]]. Historically, sodium hydroxide was obtained in an impure form from wood ashes and called soda lye. It was used to make a [[soap|soap]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 8: | Line 8: | ||
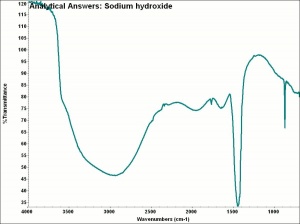
[[[SliderGallery rightalign|aaiNAOH.jpg~FTIR|sodium hydroxide.jpg~Chemical structure]]] | [[[SliderGallery rightalign|aaiNAOH.jpg~FTIR|sodium hydroxide.jpg~Chemical structure]]] | ||
| + | == Risks == | ||
| − | == | + | * Toxic by ingestion and inhalation. Highly corrosive to skin tissue. |
| + | * ThermoFisher: [https://www.fishersci.com/msds?productName=AC380210100&productDescription=SODIUM+HYDROXIDE%2C+50%25+SO+10LT&catNo=AC38021-0100&vendorId=VN00032119&storeId=10652 SDS] | ||
| + | == Physical and Chemical Properties == | ||
Soluble in water (pH is about 13 for a 0.5% solution). Soluble in glycerol, ethanol, methanol. | Soluble in water (pH is about 13 for a 0.5% solution). Soluble in glycerol, ethanol, methanol. | ||
| Line 22: | Line 25: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 318 | + | | 318 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.13 | + | | 2.13 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 34: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 1390 | + | | 1390 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 737 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 737 | ||
Latest revision as of 08:02, 2 June 2022
Description
White, deliquescent solid. Sodium hydroxide was the 8th highest-volume chemical produced in the U.S. in 1991. It is primarily used in the manufacture of chemicals, paper, soaps (Castile soap), cleaners (Drano) as well as for mercerizing cotton, and neutralizing acids. It is also used to digest cellulose in making Viscose rayon and Cellophane. Historically, sodium hydroxide was obtained in an impure form from wood ashes and called soda lye. It was used to make a Soap.
Synonyms and Related Terms
caustic soda; sodium hydrate; lye; soda lye; white caustic
Risks
- Toxic by ingestion and inhalation. Highly corrosive to skin tissue.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water (pH is about 13 for a 0.5% solution). Soluble in glycerol, ethanol, methanol.
| Composition | NaOH |
|---|---|
| CAS | 1310-73-2 |
| Melting Point | 318 C |
| Density | 2.13 g/ml |
| Molecular Weight | mol. wt. = 40.0 |
| Boiling Point | 1390 C |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 737
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Gordon Hanlon, contributed information, 1998
- The Dictionary of Paper, American Paper Institute, New York, Fourth Edition, 1980
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8772
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998