Difference between revisions of "Sodium sulfite"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | White, crystalline powder. Sodium sulfite is primarily used as a reducing agent in photographic fixers and as a replacement for 'hypo' ([ | + | White, crystalline powder. Sodium sulfite is primarily used as a reducing agent in photographic fixers and as a replacement for 'hypo' ([[sodium%20thiosulfate|sodium thiosulfate]]). It is also used for bleaching [[wool|wool]], [[straw|straw]], and [[silk|silk]]. Sodium sulfite acts as an [[antichlor%20agent|antichlor]] for the removal of [[chlorine|chlorine]] in bleached [[textile|textiles]] and [[paper|paper]], but it can leave a residual [[sulfur|sulfur]] odor. It is also used for silvering [[glass|glass]] and preserving food. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
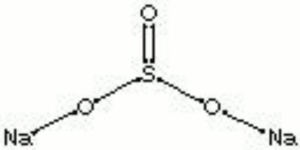
[[[SliderGallery rightalign|sodium sulfite.jpg~Chemical structure]]] | [[[SliderGallery rightalign|sodium sulfite.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| − | + | * Emits toxic fumes when heated. | |
| + | * Inhalation of powder may cause asthmatic reactions. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC310180100&productDescription=SODIUM+SULFIDE+10G&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| − | Insoluble in ethanol. | + | == Physical and Chemical Properties == |
| + | |||
| + | * Soluble in water, glycerol. | ||
| + | * Solutions smell sulfurous. | ||
| + | * Insoluble in ethanol. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 24: | Line 30: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 600 (dec) | + | | 600 C (dec) |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.633 | + | | 2.633 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 36: | Line 42: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 52: | Line 50: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8831 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8831 | ||
| − | * | + | * Photographic chemical at www.jetcity.com/~mrjones/chemdesc.htm |
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index= 1.565, 1.515 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index= 1.565, 1.515 | ||
Latest revision as of 10:29, 2 June 2022
Description
White, crystalline powder. Sodium sulfite is primarily used as a reducing agent in photographic fixers and as a replacement for 'hypo' (Sodium thiosulfate). It is also used for bleaching Wool, Straw, and Silk. Sodium sulfite acts as an antichlor for the removal of Chlorine in bleached textiles and Paper, but it can leave a residual Sulfur odor. It is also used for silvering Glass and preserving food.
Synonyms and Related Terms
sodium sulphite; disodium salt of sulfurous acid; disodium sulfite
Risks
- Emits toxic fumes when heated.
- Inhalation of powder may cause asthmatic reactions.
- ThermoFisher: SDS
Physical and Chemical Properties
- Soluble in water, glycerol.
- Solutions smell sulfurous.
- Insoluble in ethanol.
| Composition | NaSO3 |
|---|---|
| CAS | 7757-83-7 |
| Melting Point | 600 C (dec) |
| Density | 2.633 g/ml |
| Molecular Weight | mol. wt. = 126.06 |
| Refractive Index | 1.565, 1.515 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8831
- Photographic chemical at www.jetcity.com/~mrjones/chemdesc.htm
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index= 1.565, 1.515
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998