Difference between revisions of "Tetrahydrofuran"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
(→Risks) |
||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | Colorless, transparent liquid with an ether-like odor. Tetrahydrofuran (THF) is a strong solvent for natural and synthetic resins, especially [ | + | Colorless, transparent liquid with an ether-like odor. Tetrahydrofuran (THF) is a strong solvent for natural and synthetic resins, especially [[vinyl%20resin|vinyls]]. It is used in the manufacture of [[coating|coatings]], [[adhesive|adhesives]], [[magnetic%20tape|magnetic tapes]], [[printing%20ink|printing inks]], and [[cellophane|cellophane]]. Because THF is soluble in both water and organic solvents, it is chosen as an intermediate carrier in liquid chromatography solvent cycles. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
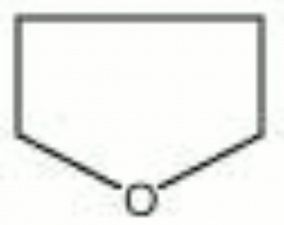
[[[SliderGallery rightalign|tetrahydrofuran.jpg~Chemical structure]]] | [[[SliderGallery rightalign|tetrahydrofuran.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Flammable. Fire risk. | ||
| + | * Toxic by ingestion and inhalation. | ||
| + | * Skin contact may cause irritation. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=BP11401&productDescription=TETRAHYDROFURAN+SEQ+1L&vendorId=VN00033897&countryCode=US&language=en SDS] | ||
| + | * EPA lists tetrahydrofuran as hazardous waste (ignitable, toxic); Concentrations over 10% must be disposed of appropriately | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
Soluble in water and organic solvents. | Soluble in water and organic solvents. | ||
| Line 22: | Line 30: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -108.4 | + | | -108.4 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.888-0.889 | + | | 0.888-0.889 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 42: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 66 | + | | 66 C |
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 47: | Line 49: | ||
[[media:download_file_137.pdf|Properties of Common Solvents]] | [[media:download_file_137.pdf|Properties of Common Solvents]] | ||
| − | + | ==Resources and Citations== | |
| − | |||
| − | == | ||
* ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 9351 | * ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 9351 | ||
Latest revision as of 13:31, 17 April 2024
Description
Colorless, transparent liquid with an ether-like odor. Tetrahydrofuran (THF) is a strong solvent for natural and synthetic resins, especially vinyls. It is used in the manufacture of coatings, adhesives, magnetic tapes, printing inks, and Cellophane. Because THF is soluble in both water and organic solvents, it is chosen as an intermediate carrier in liquid chromatography solvent cycles.
Synonyms and Related Terms
THF; diethylene oxide; tetramethylene oxide; 1,4-epoxybutane; oxacyclopentane; Oxolane
Risks
- Flammable. Fire risk.
- Toxic by ingestion and inhalation.
- Skin contact may cause irritation.
- ThermoFisher: SDS
- EPA lists tetrahydrofuran as hazardous waste (ignitable, toxic); Concentrations over 10% must be disposed of appropriately
Physical and Chemical Properties
Soluble in water and organic solvents.
| Composition | C4H8O |
|---|---|
| CAS | 109-99-9 |
| Melting Point | -108.4 C |
| Density | 0.888-0.889 g/ml |
| Molecular Weight | mol. wt. = 72.11 |
| Refractive Index | 1.4070 |
| Boiling Point | 66 C |
Comparisons
Resources and Citations
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 9351
- G.G. Hawley, The Condensed Chemical Dictionary, Reinhold Publishing, New York, 6th ed., 1961
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976