Difference between revisions of "Nylon 6"
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
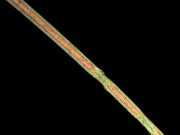
| − | [[File:65 Caprolan Nylon6 200X pol.jpg|thumb|Nylon 6]] | + | [[File:65 Caprolan Nylon6 200X pol.jpg|thumb|Nylon 6 at 200x polarized light]] |
== Description == | == Description == | ||
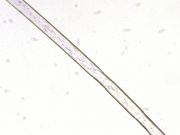
| − | + | [[File:65 Caprolan Nylon6 200X.jpg|thumb|Nylon 6 at 200x transmitted light]] | |
Nylon 6 is made by polymerizing [[caprolactam|caprolactam]] under pressure. Nylon 6 was first sold as Perlon L in 1939 by I.G.Farbenindustrie. It was produced during W.W.II for parachutes. Nylon 6 is very similar to [[nylon%206%2C6|nylon 6,6]] except that it has a greater affinity for [[dye|dyes]] and has a lower melting point. Its [[thermoplastic|thermoplastic]] fibers are strong, tough, elastic and have high gloss. They are extruded through a spinneret with a circular cross section. Nylon monofilaments are used for brushes, surgical sutures, tennis strings, and fishing lines. Nylon 6 is also used for heat-seal films because it has low water vapor transmission rates. Cellular nylon foam is made from nylon 6 for lightweight buoys and flotation products. | Nylon 6 is made by polymerizing [[caprolactam|caprolactam]] under pressure. Nylon 6 was first sold as Perlon L in 1939 by I.G.Farbenindustrie. It was produced during W.W.II for parachutes. Nylon 6 is very similar to [[nylon%206%2C6|nylon 6,6]] except that it has a greater affinity for [[dye|dyes]] and has a lower melting point. Its [[thermoplastic|thermoplastic]] fibers are strong, tough, elastic and have high gloss. They are extruded through a spinneret with a circular cross section. Nylon monofilaments are used for brushes, surgical sutures, tennis strings, and fishing lines. Nylon 6 is also used for heat-seal films because it has low water vapor transmission rates. Cellular nylon foam is made from nylon 6 for lightweight buoys and flotation products. | ||
See also [[nylon%20fiber|nylon fiber]]. | See also [[nylon%20fiber|nylon fiber]]. | ||
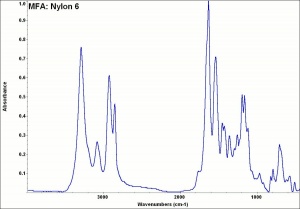
| − | + | [[[SliderGallery rightalign|MFA- Nylon 6.jpg~FTIR]]] | |
| − | [[ | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
polycaprolactam; Perlon® [I.G.Farbenindustrie]; Caprolan® [Honeywell]; Kapron; Silon; Dederon; Danamid; Nivion; Enka®; Hydrofil [Honeywell]; Powersilk [BASF]; Dorlon (later called Bayer-Perlon) [Bayer]; Bobingen (later called Hoescht-Perlon) [Hoescht] | polycaprolactam; Perlon® [I.G.Farbenindustrie]; Caprolan® [Honeywell]; Kapron; Silon; Dederon; Danamid; Nivion; Enka®; Hydrofil [Honeywell]; Powersilk [BASF]; Dorlon (later called Bayer-Perlon) [Bayer]; Bobingen (later called Hoescht-Perlon) [Hoescht] | ||
| − | [ | + | == Personal Risks == |
| + | |||
| + | * Stratasys: [http://www.hoehnplastics.com/pdf/sds-104-polyamide-6-nylon-6.pdf SDS] | ||
| − | == | + | == Physical and Chemical Properties == |
Resistant to alkalis and most organic solvents. Degraded by concentrated acids and phenol. Burns with yellow-orange flame and blue smoke; smells of burnt horn. Fiber is smooth. Cross section is circular. Tenacity = 3.8-8.3 g/denier (dry); 3.5-7.1 (wet); Elongation = 16-50% (dry); 19-55 % (wet); Moisture regain = 3.5-5.0% (dry) | Resistant to alkalis and most organic solvents. Degraded by concentrated acids and phenol. Burns with yellow-orange flame and blue smoke; smells of burnt horn. Fiber is smooth. Cross section is circular. Tenacity = 3.8-8.3 g/denier (dry); 3.5-7.1 (wet); Elongation = 16-50% (dry); 19-55 % (wet); Moisture regain = 3.5-5.0% (dry) | ||
| Line 31: | Line 32: | ||
| 1.14 | | 1.14 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
[[media:download_file_69.pdf|Properties of Synthetic Fibers]] | [[media:download_file_69.pdf|Properties of Synthetic Fibers]] | ||
| − | |||
| − | |||
== Sources Checked for Data in Record == | == Sources Checked for Data in Record == | ||
| Line 52: | Line 43: | ||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| − | * Marjory L. Joseph, ''Introductory Textile Science'', Holt, Rinehart and Winston, Fort Worth, TX, 1986 | + | * Marjory L. Joseph, ''Introductory Textile Science'', Holt, Rinehart and Winston, Fort Worth, TX, 1986, p. 135. |
| − | * J.Gordon Cook, ''Handbook of Textile Fibres:II Man-made Fibres'', Merrow Publishing Co. , Durham, England | + | * J.Gordon Cook, ''Handbook of Textile Fibres:II Man-made Fibres'', Merrow Publishing Co., Durham, England, 1984, p.261. |
* F. Kidd, ''Brushmaking Materials'', Bristish Brush Manufacturers, London, 1957 | * F. Kidd, ''Brushmaking Materials'', Bristish Brush Manufacturers, London, 1957 | ||
* Meredith Montague, contributed information, 1998 | * Meredith Montague, contributed information, 1998 | ||
| − | |||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 13:07, 19 October 2022
Description
Nylon 6 is made by polymerizing Caprolactam under pressure. Nylon 6 was first sold as Perlon L in 1939 by I.G.Farbenindustrie. It was produced during W.W.II for parachutes. Nylon 6 is very similar to Nylon 6,6 except that it has a greater affinity for dyes and has a lower melting point. Its Thermoplastic fibers are strong, tough, elastic and have high gloss. They are extruded through a spinneret with a circular cross section. Nylon monofilaments are used for brushes, surgical sutures, tennis strings, and fishing lines. Nylon 6 is also used for heat-seal films because it has low water vapor transmission rates. Cellular nylon foam is made from nylon 6 for lightweight buoys and flotation products.
See also Nylon fiber.
Synonyms and Related Terms
polycaprolactam; Perlon® [I.G.Farbenindustrie]; Caprolan® [Honeywell]; Kapron; Silon; Dederon; Danamid; Nivion; Enka®; Hydrofil [Honeywell]; Powersilk [BASF]; Dorlon (later called Bayer-Perlon) [Bayer]; Bobingen (later called Hoescht-Perlon) [Hoescht]
Personal Risks
- Stratasys: SDS
Physical and Chemical Properties
Resistant to alkalis and most organic solvents. Degraded by concentrated acids and phenol. Burns with yellow-orange flame and blue smoke; smells of burnt horn. Fiber is smooth. Cross section is circular. Tenacity = 3.8-8.3 g/denier (dry); 3.5-7.1 (wet); Elongation = 16-50% (dry); 19-55 % (wet); Moisture regain = 3.5-5.0% (dry)
| Composition | (C6H11NO)n |
|---|---|
| CAS | 25038-54-4 |
| Melting Point | 210-217 |
| Density | 1.14 |
Comparisons
Properties of Synthetic Fibers
Sources Checked for Data in Record
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Marjory L. Joseph, Introductory Textile Science, Holt, Rinehart and Winston, Fort Worth, TX, 1986, p. 135.
- J.Gordon Cook, Handbook of Textile Fibres:II Man-made Fibres, Merrow Publishing Co., Durham, England, 1984, p.261.
- F. Kidd, Brushmaking Materials, Bristish Brush Manufacturers, London, 1957
- Meredith Montague, contributed information, 1998