Difference between revisions of "Verditer"
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| (2 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | Synthetically prepared green or blue [[basic%20copper%20carbonate|basic copper carbonate]]. Verditer was an inexpensive pigment that was manufactured in the 16th century by pouring copper nitrate on calcium carbonate (whiting), followed by washing and drying. | + | Synthetically prepared copper-based green or blue [[basic%20copper%20carbonate|basic copper carbonate]]. Verditer was an inexpensive pigment that was manufactured in the 16th century as a replacement for the more expensive azurite and malachite pigments. Chemically, blue verditer is the same as azurite, while green verditer is chemically the same as malachite. In its early history of manufacture, it was made by pouring copper nitrate on calcium carbonate (whiting), followed by washing and drying. Careful control of the conditions including the temperature and concentration of the nitrate solution resulted in a blue or green color (Bristow: 18). At one time, blue verditer was manufactured on a large scale in England, and there are reports that 'the artificial blues from copper are probably more significant in medieval painting than all the rest (of the blue pigments) put together' (Thompson in Gettens and Stout: 98). Green verditer is mentioned in 17th c literature but it does not seem to have been as common as blue verditer, having a very pale color. Blue verditers were used for both distemper and oil based interior house paints, and have seen widespread use in wallpapers. See [[malachite|malachite]], and [[azurite|azurite]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | basic copper carbonate | + | basic copper carbonate, green bice, blue bice (R. Harley notes that 'blue bice' was a term for azurite in the 17th century, but by the 18th century the term referred to the synthetic analogue), Bremen blue, cendres blue, ashes blue |
| + | |||
| + | ==Properties of Blue Verditer== | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! scope="row"| Composition | ||
| + | | Cu3[OHCO3]2 | ||
| + | |- | ||
| + | ! scope="row"| Refractive Index | ||
| + | | 1.730, 1.758, 1.838 | ||
| + | |} | ||
| + | |||
| + | ==Properties of Green Verditer== | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! scope="row"| Composition | ||
| + | | Cu2[(OH)2CO3] | ||
| + | |- | ||
| + | ! scope="row"| Refractive Index | ||
| + | | 1.655, 1.18.75, 1.909 | ||
| + | |} | ||
| + | |||
| + | ==Microscopic Properties== | ||
| + | |||
| + | Blue verditer (synthetic azurite): In plane polarized light, a pale blue to blue-green color with spherulitic particles reported as approx. 5-10um in diameter. Broken spherules and fibers will also be present. In intact spherules, a dark central spot may be observed. Relief is moderate with RI>1.662. Under crossed polars, birefringence is strong with second-order interference colors masked by the (blue) body color. Extinction is incomplete, but an extinction cross may be visible in intact spherules. | ||
| + | |||
| + | Green verditer (synthetic malachite): In plane polarized light, similar to blue verditer but displaying a paler, greenish color. Spherules reported as approx. 2-10um in diameter. Broken spherules will also be present. In intact spherules, a dark central spot may be observed. Relief is moderate to low with RI>1.662. Under crossed polars, birefringence is strong with second to third-order interference colors masked by the (greenish) body color. An extinction cross may be visible in intact spherules. | ||
| + | |||
| + | ==Additional Images== | ||
| + | <gallery> | ||
| + | File:Blue verditer PPL 400x.jpg|Blue verditer, PPL, 400x | ||
| + | File:Blue verditer XPL 400x.jpg|Blue verditer, XPL, 400x | ||
| + | File:Blue bice_verditer PPL 1000x.jpg|Blue verditer, PPL, 100x | ||
| + | File:Blue bice_verditer XPL 1000x.jpg|Blue verditer, XPL, 1000x | ||
| + | File:Green verditer_Synthesized malachite PPL 400x.jpg|Green verditer (Kremer), PPL, 400x | ||
| + | File:Green verditer_Synthesized malachite XPL 400x.jpg|Green verditer (Kremer), XPL, 400x | ||
| + | </gallery> | ||
== Comparisons == | == Comparisons == | ||
[[media:download_file_493.pdf|Characteristics of Common Blue Pigments]] | [[media:download_file_493.pdf|Characteristics of Common Blue Pigments]] | ||
| + | |||
| + | ==Resources and Citations== | ||
| + | |||
| + | * ''Artists' Pigments: A Handbook of their History and Characteristics'', Ashok Roy (ed.), National Gallery of Art, Washington DC, Vol. 2, 1993 | ||
| + | |||
| + | * Bristow, I. ''Interior House-Painting Colours and Technology 1615-1840'', Yale University Press, London, 1996 | ||
| + | |||
| + | * Eastaugh, N., et. al. ''The Pigment Compendium: a Dictionary and Optical Microscopy of Historical Pigments'', Butterworth-Heinemann, London, 2008 | ||
| + | |||
| + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| + | |||
| + | * R.D. Harley, ''Artists' Pigments c. 1600-1835'', Butterworth Scientific, London, 1982 | ||
| + | |||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 11:15, 25 June 2022
Description
Synthetically prepared copper-based green or blue Basic copper carbonate. Verditer was an inexpensive pigment that was manufactured in the 16th century as a replacement for the more expensive azurite and malachite pigments. Chemically, blue verditer is the same as azurite, while green verditer is chemically the same as malachite. In its early history of manufacture, it was made by pouring copper nitrate on calcium carbonate (whiting), followed by washing and drying. Careful control of the conditions including the temperature and concentration of the nitrate solution resulted in a blue or green color (Bristow: 18). At one time, blue verditer was manufactured on a large scale in England, and there are reports that 'the artificial blues from copper are probably more significant in medieval painting than all the rest (of the blue pigments) put together' (Thompson in Gettens and Stout: 98). Green verditer is mentioned in 17th c literature but it does not seem to have been as common as blue verditer, having a very pale color. Blue verditers were used for both distemper and oil based interior house paints, and have seen widespread use in wallpapers. See Malachite, and Azurite.
Synonyms and Related Terms
basic copper carbonate, green bice, blue bice (R. Harley notes that 'blue bice' was a term for azurite in the 17th century, but by the 18th century the term referred to the synthetic analogue), Bremen blue, cendres blue, ashes blue
Properties of Blue Verditer
| Composition | Cu3[OHCO3]2 |
|---|---|
| Refractive Index | 1.730, 1.758, 1.838 |
Properties of Green Verditer
| Composition | Cu2[(OH)2CO3] |
|---|---|
| Refractive Index | 1.655, 1.18.75, 1.909 |
Microscopic Properties
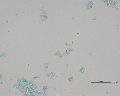
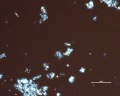
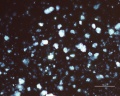
Blue verditer (synthetic azurite): In plane polarized light, a pale blue to blue-green color with spherulitic particles reported as approx. 5-10um in diameter. Broken spherules and fibers will also be present. In intact spherules, a dark central spot may be observed. Relief is moderate with RI>1.662. Under crossed polars, birefringence is strong with second-order interference colors masked by the (blue) body color. Extinction is incomplete, but an extinction cross may be visible in intact spherules.
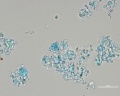
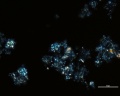
Green verditer (synthetic malachite): In plane polarized light, similar to blue verditer but displaying a paler, greenish color. Spherules reported as approx. 2-10um in diameter. Broken spherules will also be present. In intact spherules, a dark central spot may be observed. Relief is moderate to low with RI>1.662. Under crossed polars, birefringence is strong with second to third-order interference colors masked by the (greenish) body color. An extinction cross may be visible in intact spherules.
Additional Images
Comparisons
Characteristics of Common Blue Pigments
Resources and Citations
- Artists' Pigments: A Handbook of their History and Characteristics, Ashok Roy (ed.), National Gallery of Art, Washington DC, Vol. 2, 1993
- Bristow, I. Interior House-Painting Colours and Technology 1615-1840, Yale University Press, London, 1996
- Eastaugh, N., et. al. The Pigment Compendium: a Dictionary and Optical Microscopy of Historical Pigments, Butterworth-Heinemann, London, 2008
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982