Difference between revisions of "Vinyl acetate"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
(→Risks) |
||
| (One intermediate revision by the same user not shown) | |||
| Line 9: | Line 9: | ||
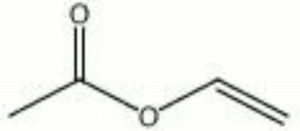
[[[SliderGallery rightalign|vinyl acetate.jpg~Chemical structure]]] | [[[SliderGallery rightalign|vinyl acetate.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Flammable. | ||
| + | * Toxic by inhalation and ingestion. | ||
| + | * Contact causes irritation. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC140840010&productDescription=VINYL+ACETATE+99%2B%25+1LT&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
Soluble in most organic solvents. Insoluble in water. | Soluble in most organic solvents. Insoluble in water. | ||
| Line 22: | Line 29: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -100.2 | + | | -100.2 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.9345 | + | | 0.9345 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 41: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 72.7 | + | | 72.7 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 49: | Line 50: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 10130 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 10130 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Vinyl_acetate (Accessed Jan. 6 2006) |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 13:08, 25 June 2022
Description
A colorless liquid used in the production of several polymers, i.e., Polyvinyl acetate, Polyvinyl alcohol, Polyvinyl butyral, and Polyvinyl chloride acetate. The process for making vinyl acetate was first patented in 1958 by Celanese.
Synonyms and Related Terms
acetic acid ethenyl ester; acetic acid vinyl ester; vinyl acetate monomor (VAM); a-acetoxyethylene
Risks
- Flammable.
- Toxic by inhalation and ingestion.
- Contact causes irritation.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in most organic solvents. Insoluble in water.
| Composition | CH3COOCH:CH2 |
|---|---|
| CAS | 108-05-4 |
| Melting Point | -100.2 C |
| Density | 0.9345 g/ml |
| Molecular Weight | mol. wt. = 86.09 |
| Refractive Index | 1.3941 |
| Boiling Point | 72.7 C |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 10130
- Wikipedia: http://en.wikipedia.org/wiki/Vinyl_acetate (Accessed Jan. 6 2006)