Difference between revisions of "Cellulose acetate"
| (16 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| + | [[File:CA gown 20101381.jpg|thumb| Wedding gown<br>MFA# 20101381]] | ||
[[File:57-75_Cellulose.Acetate.jpg|thumb|Cellulose Acetate]] | [[File:57-75_Cellulose.Acetate.jpg|thumb|Cellulose Acetate]] | ||
== Description == | == Description == | ||
| − | A general name for thermoplastic polymers composed of the acetic acid ester of cellulose. Cellulose acetate | + | A general name for thermoplastic polymers composed of the acetic acid ester of cellulose. Cellulose acetate is made by chemically modifying cotton [[linters|linters]] and/or purified [[wood pulp]] with [[sulfuric acid]], then acetylated with [[acetic acid]] and [[acetic anhydride]]. The first portion of the reaction produces cellulose triacetate; cellulose diacetate is obtained by partial hydrolysis to replace some of the acetyl groups with hydroxyl groups. Cellulose diacetate is commonly called acetate while the cellulose triacetate is called triacetate. Plasticizers, such as glycol phthalate, tributyl phosphate, dimethyl phthalate, diethyl phthalate or [[dibutyl phthalate]] are added to cellulose acetate to increase flexibility. The plasticizers also help to make the material less flammable (Richardson 2014). |
| − | Cellulose acetate | + | Cellulose acetate was first developed by Schutzenberger in 1865, but wasn't patented until 1894. In 1908, it was introduced as 'safety' film by Eastman Kodak because it showed similar properties and uses to cellulose nitrate but was less flammable. By 1910, with production by Dreyfus in France, cellulose acetate began replacing cellulose nitrate film. Cellulose acetate fibers for use in clothing came later; they were manufactured commercially in Great Britain in 1919 (Celanese®). Today, cellulose acetate resins are still used in lacquers, photographic film, transparent sheeting and as fibers. |
See also [[acetate fiber]], [[cellulose diacetate]], [[cellulose triacetate]], and [[cellulose acetate butyrate]]. | See also [[acetate fiber]], [[cellulose diacetate]], [[cellulose triacetate]], and [[cellulose acetate butyrate]]. | ||
| Line 12: | Line 13: | ||
CA; acétate de cellulose (Fr.); Celluloseacetat (Deut.); acetato de celulosa (Esp.); acetato di cellulosa (It.); acetato de celulose (Port.); safety film; secondary acetate | CA; acétate de cellulose (Fr.); Celluloseacetat (Deut.); acetato de celulosa (Esp.); acetato di cellulosa (It.); acetato de celulose (Port.); safety film; secondary acetate | ||
| − | Examples: Celanese [British Celanese]; Kodacel [Eastman Kodak]; Tenite; Similoid; | + | Examples: Celanese [British Celanese]; Kodacel [Eastman Kodak]; Tenite; Similoid; Lumarith |
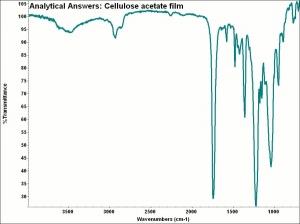
[[[SliderGallery rightalign|aaiCELLACET.jpg~FTIR]]] | [[[SliderGallery rightalign|aaiCELLACET.jpg~FTIR]]] | ||
| + | |||
== Applications == | == Applications == | ||
| − | * Photographic film | + | * Photographic film, animations cels, transparent sheeting |
| − | * | + | * Eyeglass frames, tool handles |
* Lacquers and coatings | * Lacquers and coatings | ||
| − | * | + | * Textiles and fibers |
| + | * Cigarette filters, wound dressings, personal hygiene products | ||
== Personal Risks == | == Personal Risks == | ||
| + | Pure material burns readily; plasticized material also burns but takes longer to ignite. | ||
| + | |||
| + | ThermoFisher: [https://www.fishersci.com/shop/msdsproxy?productName=AC382310050&productDescription=CELLULOSE+MICROCRYSTALLI+5KG&catNo=AC382310050&vendorId=VN00032119&storeId=10652 SDS] | ||
== Collection Risks == | == Collection Risks == | ||
| − | Degradation produces volatile acetic acid. | + | * Degrades in sunlight, heat, and high humidity. |
| + | * Degradation produces volatile acetic acid; Should be stored cold and away from other objects. | ||
| + | * Plasticizer migration may result in oily or sticky surfaces. | ||
| + | * Resistant to insects, slowly degraded by microorganisms. | ||
== Physical and Chemical Properties == | == Physical and Chemical Properties == | ||
| Line 34: | Line 43: | ||
* Density = 1.25-1.35 | * Density = 1.25-1.35 | ||
* Refractive Index = 1.48 (pure) | * Refractive Index = 1.48 (pure) | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 53: | Line 50: | ||
[[media:download_file_325.pdf|General Characteristics of Polymers]] | [[media:download_file_325.pdf|General Characteristics of Polymers]] | ||
| − | + | ==Resources and Citations== | |
| − | + | * Catherine Stephens, AIC Plastics Panel, Submitted information, 2020. | |
| − | == | + | * Jean Tetreault ''Products Used in Preventive Conservation'' CCI, December 2017. [https://www.researchgate.net/publication/323153775_Products_Used_in_Preventive_Conservation Link] |
| − | + | * Museum Handbook, Part 1. Museums Collections. Web Edition. Appendix M. Management of Cellulose Nitrate and Cellulose Acetate Films, NPS, 2001. [http://www.cr.nps.gov/museum/publications/MHI/AppendM.pdf http://www.cr.nps.gov/museum/publications/MHI/AppendM.pdf] | |
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| − | |||
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| − | |||
* Rosalie Rosso King, ''Textile Identification, Conservation, and Preservation'', Noyes Publications, Park Ridge, NJ, 1985 | * Rosalie Rosso King, ''Textile Identification, Conservation, and Preservation'', Noyes Publications, Park Ridge, NJ, 1985 | ||
| − | |||
* Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | ||
| − | |||
* C.V.Horie, ''Materials for Conservation'', Butterworth-Heineman, London, 1997 | * C.V.Horie, ''Materials for Conservation'', Butterworth-Heineman, London, 1997 | ||
| − | |||
* Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | ||
| − | |||
* Pam Hatchfield, ''Pollutants in the Museum Environment'', Archetype Press, London, 2002 | * Pam Hatchfield, ''Pollutants in the Museum Environment'', Archetype Press, London, 2002 | ||
| − | |||
* Marjory L. Joseph, ''Introductory Textile Science'', Holt, Rinehart and Winston, Fort Worth, TX, 1986 | * Marjory L. Joseph, ''Introductory Textile Science'', Holt, Rinehart and Winston, Fort Worth, TX, 1986 | ||
| − | |||
* John S. Mills, Raymond White, ''The Organic Chemistry of Museum Objects'', Butterworth Heineman, London, 2nd ed., 1994 | * John S. Mills, Raymond White, ''The Organic Chemistry of Museum Objects'', Butterworth Heineman, London, 2nd ed., 1994 | ||
| − | |||
* M.Kaufman, ''The First Century of Plastics'', The Plastics and Rubber Institute, London, 1963 | * M.Kaufman, ''The First Century of Plastics'', The Plastics and Rubber Institute, London, 1963 | ||
| − | + | * Wikipedia: http://en.wikipedia.org/wiki/Cellulose_acetate (Accessed Jan. 6 2006) - gives following dates: resin-1865, film-1934, fiber-1924 | |
| − | * Wikipedia | ||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p.169 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p.169 | ||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| − | |||
* Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 | * Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 | ||
| − | |||
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | |||
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
| − | |||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | |||
* Sharon Blank, An introduction to plastics and rubbers in collections, ''Studies in Conservation'', 35, 53-63, 1990 | * Sharon Blank, An introduction to plastics and rubbers in collections, ''Studies in Conservation'', 35, 53-63, 1990 | ||
| − | |||
* Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 | * Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 | ||
| − | |||
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
| − | + | * History of Plastics: www.nswpmith.com.au/historyofplastics.html | |
| − | * | ||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 14:25, 2 October 2024
Description
A general name for thermoplastic polymers composed of the acetic acid ester of cellulose. Cellulose acetate is made by chemically modifying cotton Linters and/or purified Wood pulp with Sulfuric acid, then acetylated with Acetic acid and Acetic anhydride. The first portion of the reaction produces cellulose triacetate; cellulose diacetate is obtained by partial hydrolysis to replace some of the acetyl groups with hydroxyl groups. Cellulose diacetate is commonly called acetate while the cellulose triacetate is called triacetate. Plasticizers, such as glycol phthalate, tributyl phosphate, dimethyl phthalate, diethyl phthalate or Dibutyl phthalate are added to cellulose acetate to increase flexibility. The plasticizers also help to make the material less flammable (Richardson 2014).
Cellulose acetate was first developed by Schutzenberger in 1865, but wasn't patented until 1894. In 1908, it was introduced as 'safety' film by Eastman Kodak because it showed similar properties and uses to cellulose nitrate but was less flammable. By 1910, with production by Dreyfus in France, cellulose acetate began replacing cellulose nitrate film. Cellulose acetate fibers for use in clothing came later; they were manufactured commercially in Great Britain in 1919 (Celanese®). Today, cellulose acetate resins are still used in lacquers, photographic film, transparent sheeting and as fibers.
See also Acetate fiber, Cellulose diacetate, Cellulose triacetate, and Cellulose acetate butyrate.
Synonyms and Related Terms
CA; acétate de cellulose (Fr.); Celluloseacetat (Deut.); acetato de celulosa (Esp.); acetato di cellulosa (It.); acetato de celulose (Port.); safety film; secondary acetate
Examples: Celanese [British Celanese]; Kodacel [Eastman Kodak]; Tenite; Similoid; Lumarith
Applications
- Photographic film, animations cels, transparent sheeting
- Eyeglass frames, tool handles
- Lacquers and coatings
- Textiles and fibers
- Cigarette filters, wound dressings, personal hygiene products
Personal Risks
Pure material burns readily; plasticized material also burns but takes longer to ignite.
ThermoFisher: SDS
Collection Risks
- Degrades in sunlight, heat, and high humidity.
- Degradation produces volatile acetic acid; Should be stored cold and away from other objects.
- Plasticizer migration may result in oily or sticky surfaces.
- Resistant to insects, slowly degraded by microorganisms.
Physical and Chemical Properties
- Resistant to dilute alkalis and dry-cleaning solvents.
- Degrades in acids and concentrated alkalis.
- Soluble in acetone, phenol and chloroform.
- Produces tiny sparks when burnt and gives faint odor of acetic acid.
- Plasticized cellulose acetate: refractive index = 1.48-1.51
- Melting Point = 232
- Density = 1.25-1.35
- Refractive Index = 1.48 (pure)
Comparisons
Physical Properties for Selected Thermoplastic Resins
General Characteristics of Polymers
Resources and Citations
- Catherine Stephens, AIC Plastics Panel, Submitted information, 2020.
- Jean Tetreault Products Used in Preventive Conservation CCI, December 2017. Link
- Museum Handbook, Part 1. Museums Collections. Web Edition. Appendix M. Management of Cellulose Nitrate and Cellulose Acetate Films, NPS, 2001. http://www.cr.nps.gov/museum/publications/MHI/AppendM.pdf
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Rosalie Rosso King, Textile Identification, Conservation, and Preservation, Noyes Publications, Park Ridge, NJ, 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- C.V.Horie, Materials for Conservation, Butterworth-Heineman, London, 1997
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- Marjory L. Joseph, Introductory Textile Science, Holt, Rinehart and Winston, Fort Worth, TX, 1986
- John S. Mills, Raymond White, The Organic Chemistry of Museum Objects, Butterworth Heineman, London, 2nd ed., 1994
- M.Kaufman, The First Century of Plastics, The Plastics and Rubber Institute, London, 1963
- Wikipedia: http://en.wikipedia.org/wiki/Cellulose_acetate (Accessed Jan. 6 2006) - gives following dates: resin-1865, film-1934, fiber-1924
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p.169
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Sharon Blank, An introduction to plastics and rubbers in collections, Studies in Conservation, 35, 53-63, 1990
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- History of Plastics: www.nswpmith.com.au/historyofplastics.html