Difference between revisions of "Linseed oil"
| (9 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:1993.539-SC78369.jpg|thumb|]] | + | [[File:1993.539-SC78369.jpg|thumb|Oil and graphite<br>MFA# 1993.539]] |
== Description == | == Description == | ||
| + | [[File:52-70_CP.Linseed.Oil_canvas.jpg|thumb|Linseed Oil on canvas (visible light left; UV light right)]] | ||
| + | A drying oil used in artist paints that is obtained from the seeds of the common flax (''Linum usitatissimum'') plant. Linseed oil contains the following fatty acids: [[linolenic acid|linolenic]] (48-60%), [[oleic acid|oleic]] (14-24%), [[linoleic acid|linoleic]] (14-19%), [[palmitic acid|palmitic]] (6-7%), and [[stearic acid|stearic]] (3-6%) (Serpico and White 2000). The drying property is due to the unsaturated bonds in the linoleic and linolenic groups. Linseed oil is the most important and largely used oil for paints and varnishes. It produces a hard, insoluble film when it dries. The yellow-gold color oil is commercially extracted by various methods. The seeds can be crushed in hydraulic or screw-type presses to produce cold-pressed oil. The same process performed on steam-heated seeds produces hot-pressed oil. Cold-pressing is a less efficient manner for extraction, but it produces a higher quality artist paint. Many types of aging, refining, and bleaching procedures have been used to purify the oil and make it dry faster. | ||
| − | + | Linseed oil is used in: | |
| − | + | * [[paint|Paints]]: As a paint medium, oil is fluid, transparent and glossy; pigmented linseed oil was used for artist paintings and indoor woodwork | |
| − | [[ | + | * [[varnish|Varnishes]]: As a wood varnish, linseed oil dries slowly and shrinks; it turns yellowish and darkens with age |
| + | * [[printing ink|Printing inks]]: An early printing ink contained linseed oil, turpentine and soot; More recent textile inks also contain linseed oil [Gamblin] | ||
| + | * [[synthetic resin|Synthetic resins]]: Often used with alkyd resins as paints and varnishes. | ||
| + | * [[oilcloth]]: A waterproof fabric made by coating cotton or linen with linseed oil; also called enameled cloth | ||
| + | * [[linoleum]]: Linseed oil is combined with wood dust, cork particles and other materials to manufacture linoleum | ||
| + | * [[Putty]]s: As a putty, linseed oil is combined with calk to seal glass windows; they can later be painted over | ||
| + | * [[Soap|Soaps]]: Can clean the surface of wood while adding some protection | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | ''Linum usitatissimum'' | + | ''Linum usitatissimum''; huile de lin (Fr.); Leinöl (Deut.); aceite de lino (Esp.); aceite de linaza (Esp.); olio di lino (It); lijnzaadolie (Ned.); olej lniany (Pol.); Óleo de linhaça (Port.); linoljan (Sven.); flaxseed oil; flax seed oil; flax oil; linum oil |
| − | Types include: raw; cold-pressed; refined; stand oil; blown oil; bodied oil; boiled oil; sun-refined oil; sun-bleached oil; double boiled oil | + | Types include: raw; cold-pressed; refined; stand oil; blown oil; bodied oil; boiled oil; sun-refined oil; sun-bleached oil; double boiled oil; burnt plate oil |
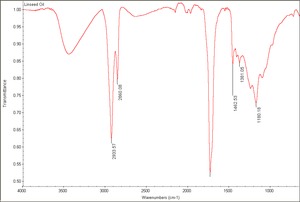
[[[SliderGallery rightalign|Linseed Oil.TIF~FTIR (MFA)]]] | [[[SliderGallery rightalign|Linseed Oil.TIF~FTIR (MFA)]]] | ||
| + | |||
== Risks == | == Risks == | ||
| − | Contact may cause allergic reaction. Aspiration hazard if swallowed. | + | * Contact may cause allergic reaction. |
| + | * Aspiration hazard if swallowed. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/71365.htm MSDS] | ||
| + | == Physical and Chemical Properties == | ||
| − | + | * As a liquid, it is soluble in ether, chloroform, carbon disulfide, ligroin and turpentine. | |
| − | + | * When dry, it is insoluble in most solvents. | |
| − | + | * Saponification number = 190-193. | |
| − | As a liquid, it is soluble in ether, chloroform, carbon disulfide, ligroin and turpentine. When dry, it is insoluble in most solvents. Saponification number = 190-193. Iodine number = 170-195. Acid number = 1-8. | + | * Iodine number = 170-195. |
| + | * Acid number = 1-8. | ||
| + | * CAS = 8001-26-1 | ||
| + | * Melting Point =-24.0 C | ||
| + | * Density = 0.921-0.936 g/ml | ||
| + | * Refractive Index = 1.48 -1.49 | ||
{| class="wikitable" | {| class="wikitable" | ||
| + | |+Content of Fatty Acid of the Drying Oil after Hydrolysis (Phenacyl Bromide Esters % w/w Total Fatty Acid) (from Tarola et. al 2012) | ||
|- | |- | ||
| − | ! | + | !Drying oil!!Linolenic acid<br>C18:3 % ± SD!!Linoleic acid<br>C18:2 % ± SD!!Myristic acid<br>C14:0 % ± SD!!Palmitic acid<br>C16:0 % ± SD!!Oleic acid<br>C18:1 % ± SD!!Stearic acid<br>C18:0 % ± SD |
| − | |||
|- | |- | ||
| − | ! | + | !Linseed oil |
| − | | | + | |58 ± 1.2 ||16 ± 0.8 ||–||7 ± 1.1 ||16 ± 1.8||3 ± 0.1 |
|- | |- | ||
| − | ! | + | !Walnut oil |
| − | | 0. | + | |8 ± 0.3||72 ± 1.5||–||6 ± 0.6||12 ± 1.1||2 ± 0.2 |
|- | |- | ||
| − | ! | + | !Poppy seed oil |
| − | | | + | |8 ± 0.7||70 ± 0.8||–||9 ± 0.5||10 ± 0.4||3 ± 0.2 |
|} | |} | ||
== Resources and Citations == | == Resources and Citations == | ||
| − | + | * Tarola, A.M., A.M. Girelli, S. Lorusso, "High Performance Liquid Chromatography Determination of Fatty Acids in Drying Oils Following Lipase Action", ''Journal of Chromatographic Science'', Vol. 50(4), April 2012, Pages 294–300. | |
| + | * Mills J.. "Composition and identification of dried oil film: The gas-chromatographic examination of paint media", ''Studies in Conservation'', 1966, vol. 11 (pg. 92-106) Part I. Fatty acid. | ||
* Jorrit van den Berg, ''Analytical chemical studies on traditional linseed oil paints'', MOLART 2002, available from Archetype Publications, London. | * Jorrit van den Berg, ''Analytical chemical studies on traditional linseed oil paints'', MOLART 2002, available from Archetype Publications, London. | ||
| − | |||
* M.Serpico, R.White, "Oil, fat and wax" in ''Ancient Egyptian Materials and Technology'', P.Nicholson, I.Shaw (eds.), Cambridge University Press, 2000, p. 390-429. | * M.Serpico, R.White, "Oil, fat and wax" in ''Ancient Egyptian Materials and Technology'', P.Nicholson, I.Shaw (eds.), Cambridge University Press, 2000, p. 390-429. | ||
| − | |||
* J.S. Mills, R.White, ''The Organic Chemistry of Museum Objects'', Butterworth Heinemann, London, 1994. | * J.S. Mills, R.White, ''The Organic Chemistry of Museum Objects'', Butterworth Heinemann, London, 1994. | ||
| − | |||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5335 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5335 | ||
| − | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Linseed." Accessed 14 Apr. 2004. | |
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Linseed." Accessed 14 Apr. 2004 | ||
| − | |||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: linolenic=36.4-40.3%; linoleic=37.9-45.0%; oleic=13.2-16.0%; stearic and palmitic=4.8-9.0% | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: linolenic=36.4-40.3%; linoleic=37.9-45.0%; oleic=13.2-16.0%; stearic and palmitic=4.8-9.0% | ||
| − | |||
* Reed Kay, ''The Painter's Guide To Studio Methods and Materials'', Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983 | * Reed Kay, ''The Painter's Guide To Studio Methods and Materials'', Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983 | ||
| − | |||
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| − | |||
* Robert Feller, Nathan Stolow, Elizabeth Jones, ''On Picture Varnishes and Their Solvents'', National Gallery of Art, Washington DC, 1985 | * Robert Feller, Nathan Stolow, Elizabeth Jones, ''On Picture Varnishes and Their Solvents'', National Gallery of Art, Washington DC, 1985 | ||
| − | |||
* Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | ||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| − | |||
* Pam Hatchfield, ''Pollutants in the Museum Environment'', Archetype Press, London, 2002 | * Pam Hatchfield, ''Pollutants in the Museum Environment'', Archetype Press, London, 2002 | ||
| − | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | |
| − | * Art and Architecture Thesaurus Online, | ||
| − | |||
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: melting point = -24.0, density=0.938, ref. index = 1.4782, iodine value=1785.7, saponification value = 190.3 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: melting point = -24.0, density=0.938, ref. index = 1.4782, iodine value=1785.7, saponification value = 190.3 | ||
| − | + | * Wikipedia: [https://en.wikipedia.org/wiki/Linseed_oil Linseed oil] Accessed March 2025 | |
| − | + | * Gamblin Artist Colors: [https://www.imcclains.com/catalog/ink/GamblinTextileInk.html Textile ink] | |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 07:28, 20 March 2025
Description
A drying oil used in artist paints that is obtained from the seeds of the common flax (Linum usitatissimum) plant. Linseed oil contains the following fatty acids: linolenic (48-60%), oleic (14-24%), linoleic (14-19%), palmitic (6-7%), and stearic (3-6%) (Serpico and White 2000). The drying property is due to the unsaturated bonds in the linoleic and linolenic groups. Linseed oil is the most important and largely used oil for paints and varnishes. It produces a hard, insoluble film when it dries. The yellow-gold color oil is commercially extracted by various methods. The seeds can be crushed in hydraulic or screw-type presses to produce cold-pressed oil. The same process performed on steam-heated seeds produces hot-pressed oil. Cold-pressing is a less efficient manner for extraction, but it produces a higher quality artist paint. Many types of aging, refining, and bleaching procedures have been used to purify the oil and make it dry faster.
Linseed oil is used in:
- Paints: As a paint medium, oil is fluid, transparent and glossy; pigmented linseed oil was used for artist paintings and indoor woodwork
- Varnishes: As a wood varnish, linseed oil dries slowly and shrinks; it turns yellowish and darkens with age
- Printing inks: An early printing ink contained linseed oil, turpentine and soot; More recent textile inks also contain linseed oil [Gamblin]
- Synthetic resins: Often used with alkyd resins as paints and varnishes.
- Oilcloth: A waterproof fabric made by coating cotton or linen with linseed oil; also called enameled cloth
- Linoleum: Linseed oil is combined with wood dust, cork particles and other materials to manufacture linoleum
- Puttys: As a putty, linseed oil is combined with calk to seal glass windows; they can later be painted over
- Soaps: Can clean the surface of wood while adding some protection
Synonyms and Related Terms
Linum usitatissimum; huile de lin (Fr.); Leinöl (Deut.); aceite de lino (Esp.); aceite de linaza (Esp.); olio di lino (It); lijnzaadolie (Ned.); olej lniany (Pol.); Óleo de linhaça (Port.); linoljan (Sven.); flaxseed oil; flax seed oil; flax oil; linum oil
Types include: raw; cold-pressed; refined; stand oil; blown oil; bodied oil; boiled oil; sun-refined oil; sun-bleached oil; double boiled oil; burnt plate oil
Risks
- Contact may cause allergic reaction.
- Aspiration hazard if swallowed.
- Fisher Scientific: MSDS
Physical and Chemical Properties
- As a liquid, it is soluble in ether, chloroform, carbon disulfide, ligroin and turpentine.
- When dry, it is insoluble in most solvents.
- Saponification number = 190-193.
- Iodine number = 170-195.
- Acid number = 1-8.
- CAS = 8001-26-1
- Melting Point =-24.0 C
- Density = 0.921-0.936 g/ml
- Refractive Index = 1.48 -1.49
| Drying oil | Linolenic acid C18:3 % ± SD |
Linoleic acid C18:2 % ± SD |
Myristic acid C14:0 % ± SD |
Palmitic acid C16:0 % ± SD |
Oleic acid C18:1 % ± SD |
Stearic acid C18:0 % ± SD |
|---|---|---|---|---|---|---|
| Linseed oil | 58 ± 1.2 | 16 ± 0.8 | – | 7 ± 1.1 | 16 ± 1.8 | 3 ± 0.1 |
| Walnut oil | 8 ± 0.3 | 72 ± 1.5 | – | 6 ± 0.6 | 12 ± 1.1 | 2 ± 0.2 |
| Poppy seed oil | 8 ± 0.7 | 70 ± 0.8 | – | 9 ± 0.5 | 10 ± 0.4 | 3 ± 0.2 |
Resources and Citations
- Tarola, A.M., A.M. Girelli, S. Lorusso, "High Performance Liquid Chromatography Determination of Fatty Acids in Drying Oils Following Lipase Action", Journal of Chromatographic Science, Vol. 50(4), April 2012, Pages 294–300.
- Mills J.. "Composition and identification of dried oil film: The gas-chromatographic examination of paint media", Studies in Conservation, 1966, vol. 11 (pg. 92-106) Part I. Fatty acid.
- Jorrit van den Berg, Analytical chemical studies on traditional linseed oil paints, MOLART 2002, available from Archetype Publications, London.
- M.Serpico, R.White, "Oil, fat and wax" in Ancient Egyptian Materials and Technology, P.Nicholson, I.Shaw (eds.), Cambridge University Press, 2000, p. 390-429.
- J.S. Mills, R.White, The Organic Chemistry of Museum Objects, Butterworth Heinemann, London, 1994.
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5335
- Encyclopedia Britannica, http://www.britannica.com Comment: "Linseed." Accessed 14 Apr. 2004.
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: linolenic=36.4-40.3%; linoleic=37.9-45.0%; oleic=13.2-16.0%; stearic and palmitic=4.8-9.0%
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Robert Feller, Nathan Stolow, Elizabeth Jones, On Picture Varnishes and Their Solvents, National Gallery of Art, Washington DC, 1985
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: melting point = -24.0, density=0.938, ref. index = 1.4782, iodine value=1785.7, saponification value = 190.3
- Wikipedia: Linseed oil Accessed March 2025
- Gamblin Artist Colors: Textile ink