Difference between revisions of "Brochantite"
Jump to navigation
Jump to search
| (2 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | A vivid green mineral composed of [[copper sulfate, tribasic|tribasic copper sulfate]]. Brochantite was discovered by French grologist, A.J.M Brochant de Villiers. It is a secondary mineral found in oxidized zones of [[copper]] deposits in Arizona, Chile, Mexico, and Russia. The mineral also forms as a bright green corrosion product on | + | A vivid green mineral composed of [[copper sulfate, tribasic|tribasic copper sulfate]]. Brochantite was discovered by French grologist, A.J.M Brochant de Villiers. It is a secondary mineral found in oxidized zones of [[copper]] deposits in Arizona, Chile, Mexico, and Russia. The mineral also forms as a bright green corrosion product on copper and [[bronze]] artifacts exposed to [[sulfur]] containing pollution. Brochantite is considered a stable patina, but may indicate pit formation for outdoor leaded bronzes due to the formation of a soluble lead sulfate. |
See also [[antlerite]]. | See also [[antlerite]]. | ||
| Line 10: | Line 10: | ||
tribasic copper sulfate; Brochantit (Deut.); brochantite (Fr., It.); brocantita (Esp.); brochantiet (Ned.); brochantite (Port.) | tribasic copper sulfate; Brochantit (Deut.); brochantite (Fr., It.); brocantita (Esp.); brochantiet (Ned.); brochantite (Port.) | ||
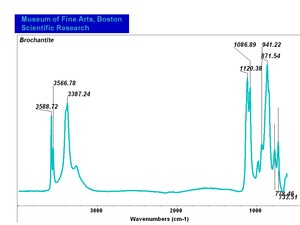
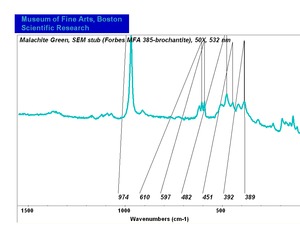
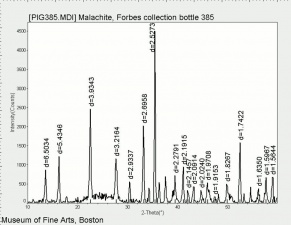
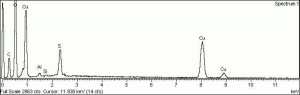
| − | [[[SliderGallery rightalign|Brochantite.TIF~FTIR (MFA)|Brochantite Raman MFA 385.TIF~Raman (MFA)|PIG385.jpg~XRD|f385sem.jpg~SEM|f385edsbw.jpg~EDS]]] | + | [[[SliderGallery rightalign|Brochantite.TIF~FTIR (MFA)|Brochantite Raman MFA 385.TIF~Raman (MFA)|PIG385.jpg~XRD (MFA)|f385sem.jpg~SEM (MFA)|f385edsbw.jpg~EDS (MFA)]]] |
== Physical and Chemical Properties == | == Physical and Chemical Properties == | ||
| − | Needle-like prisms in orthorhombic crystalline system. | + | * Needle-like prisms in orthorhombic crystalline system. |
| − | + | * Streak = pale green. | |
| − | Streak = pale green. | + | * Luster = vitreous. |
| + | * Perfect cleavage in one direction. | ||
| + | * Fracture = conchoidal | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 27: | Line 29: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 3.9-4.0 | + | | 3.9-4.0 g/ml |
|} | |} | ||
Latest revision as of 13:59, 6 December 2022
Description
A vivid green mineral composed of tribasic copper sulfate. Brochantite was discovered by French grologist, A.J.M Brochant de Villiers. It is a secondary mineral found in oxidized zones of Copper deposits in Arizona, Chile, Mexico, and Russia. The mineral also forms as a bright green corrosion product on copper and Bronze artifacts exposed to Sulfur containing pollution. Brochantite is considered a stable patina, but may indicate pit formation for outdoor leaded bronzes due to the formation of a soluble lead sulfate.
See also Antlerite.
Synonyms and Related Terms
tribasic copper sulfate; Brochantit (Deut.); brochantite (Fr., It.); brocantita (Esp.); brochantiet (Ned.); brochantite (Port.)
Physical and Chemical Properties
- Needle-like prisms in orthorhombic crystalline system.
- Streak = pale green.
- Luster = vitreous.
- Perfect cleavage in one direction.
- Fracture = conchoidal
| Composition | Cu4SO4(OH)6 |
|---|---|
| Mohs Hardness | 3.5 - 4.0 |
| Density | 3.9-4.0 g/ml |
Resources and Citations
- Mineralogy Database: Brochantite
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Thomas Gregory, The Condensed Chemical Dictionary, Reinhold Publishing, New York, 3rd ed., 1942
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- Wikipedia: http://en.wikipedia.org/wiki/Brochantite (Accessed Sept. 2, 2005)