Difference between revisions of "Ethylene glycol"
| (One intermediate revision by the same user not shown) | |||
| Line 2: | Line 2: | ||
A clear, colorless, syrupy liquid that is the simplest glycol in its homologous series. While ethylene glycol has a sweet taste, it is extremely poisonous and should not be ingested. Ethylene glycol lowers the freezing point of water and is widely used in antifreeze formulations. Additionally, ethylene glycol is [[hygroscopic]] and is used as a combined [[humectant]], [[solvent]], and [[plasticizer]] in inks, paints, plastics, textiles, and leathers. It is also used as a component to synthesize explosives, alkyd resins, plasticizers, synthetic waxes, and synthetic fibers ([[Dacron]], [[Terylene]]). | A clear, colorless, syrupy liquid that is the simplest glycol in its homologous series. While ethylene glycol has a sweet taste, it is extremely poisonous and should not be ingested. Ethylene glycol lowers the freezing point of water and is widely used in antifreeze formulations. Additionally, ethylene glycol is [[hygroscopic]] and is used as a combined [[humectant]], [[solvent]], and [[plasticizer]] in inks, paints, plastics, textiles, and leathers. It is also used as a component to synthesize explosives, alkyd resins, plasticizers, synthetic waxes, and synthetic fibers ([[Dacron]], [[Terylene]]). | ||
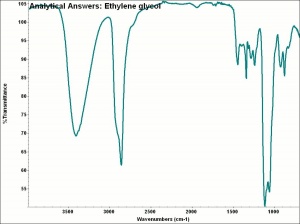
| − | + | [[[SliderGallery rightalign|aaiETHYLGLYCOL.jpg~FTIR|ethylene glycol.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
1,2-ethanediol; ethylene alcohol; glycol | 1,2-ethanediol; ethylene alcohol; glycol | ||
| − | |||
== Risks == | == Risks == | ||
| − | Toxic by ingestion and inhalation causing nervous system stimulation followed by coma and death. Lethal dose is about 100 ml. | + | * Toxic by ingestion and inhalation causing nervous system stimulation followed by coma and death. Lethal dose is about 100 ml. |
| − | + | * Combustible. Flash point = 111 C (232 F) | |
| − | Combustible. Flash point = 111 C (232 F) | + | * ThermoFisher: [https://www.fishersci.com/msdsproxy%3FproductName%3DE1774%26productDescription%3DETHYLENE%2BGLYCOL%2BLABORATORY%2B4L%26catNo%3DE177-4%2B%26vendorId%3DVN00033897%26storeId%3D10652 SDS] |
| − | |||
| − | |||
== Physical and Chemical Properties == | == Physical and Chemical Properties == | ||
| Line 29: | Line 26: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -13.5 | + | | -13.5 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.1155 | + | | 1.1155 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 41: | Line 38: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 197.2 | + | | 197.2 C |
|} | |} | ||
| − | |||
== Comparisons == | == Comparisons == | ||
Latest revision as of 15:40, 5 August 2022
Description
A clear, colorless, syrupy liquid that is the simplest glycol in its homologous series. While ethylene glycol has a sweet taste, it is extremely poisonous and should not be ingested. Ethylene glycol lowers the freezing point of water and is widely used in antifreeze formulations. Additionally, ethylene glycol is Hygroscopic and is used as a combined Humectant, Solvent, and Plasticizer in inks, paints, plastics, textiles, and leathers. It is also used as a component to synthesize explosives, alkyd resins, plasticizers, synthetic waxes, and synthetic fibers (Dacron, Terylene).
Synonyms and Related Terms
1,2-ethanediol; ethylene alcohol; glycol
Risks
- Toxic by ingestion and inhalation causing nervous system stimulation followed by coma and death. Lethal dose is about 100 ml.
- Combustible. Flash point = 111 C (232 F)
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water, ethanol, acetone, acetic acid, ketones and aldehydes. Insoluble in aromatic and chlorinated hydrocarbons.
| Composition | HOCH2CH2OH |
|---|---|
| CAS | 107-21-1 |
| Melting Point | -13.5 C |
| Density | 1.1155 g/ml |
| Molecular Weight | mol. wt. = 62.1 |
| Refractive Index | 1.429 |
| Boiling Point | 197.2 C |
Comparisons
Resources and Citations
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Hoechst Celanese Corporation, Dictionary of Fiber & Textile Technology (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 3844
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index =1.429