Difference between revisions of "Benzyl alcohol"
Jump to navigation
Jump to search
(→Risks) |
|||
| (2 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
A clear, colorless, liquid. Benzyl alcohol is used as a solvent for [[dye|dyes]], [[gelatin]], [[casein]], [[cellulose acetate]], [[wax|waxes]], and [[shellac]]. It is also used for mounting microscope slides and as a reagent in the developing baths for color movie films. In conservation, benzyl alcohol has been added to gelled soaps as a [[wetting agent]]. | A clear, colorless, liquid. Benzyl alcohol is used as a solvent for [[dye|dyes]], [[gelatin]], [[casein]], [[cellulose acetate]], [[wax|waxes]], and [[shellac]]. It is also used for mounting microscope slides and as a reagent in the developing baths for color movie films. In conservation, benzyl alcohol has been added to gelled soaps as a [[wetting agent]]. | ||
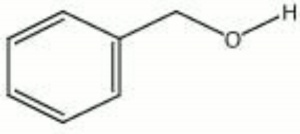
| − | + | [[[SliderGallery rightalign|benzyl alcohol.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
alpha-hydroxytoluene; benzoyl alcohol; phenyl methanol; phenyl carbinol; benzenemethanol | alpha-hydroxytoluene; benzoyl alcohol; phenyl methanol; phenyl carbinol; benzenemethanol | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Combustible. | ||
| + | * Skin contact causes irritation. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=A396500&productDescription=BENZYL+ALCOHOL+CERTIFIED+500ML&vendorId=VN00033897&countryCode=US&language=en SDS] | ||
| + | * EPA lists benzyl alcohol as hazardous waste due to ignitability; concentrations over 10% must be disposed of appropriately | ||
| − | == | + | ==Physical and Chemical Properties== |
Miscible with ethanol, ether, chloroform. Slightly soluble in water. | Miscible with ethanol, ether, chloroform. Slightly soluble in water. | ||
| Line 22: | Line 27: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -15.19 | + | | -15.19 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.040-1.050 | + | | 1.040-1.050 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 39: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 204.7 | + | | 204.7 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 97 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 97 | ||
Latest revision as of 12:23, 17 April 2024
Description
A clear, colorless, liquid. Benzyl alcohol is used as a solvent for dyes, Gelatin, Casein, Cellulose acetate, waxes, and Shellac. It is also used for mounting microscope slides and as a reagent in the developing baths for color movie films. In conservation, benzyl alcohol has been added to gelled soaps as a Wetting agent.
Synonyms and Related Terms
alpha-hydroxytoluene; benzoyl alcohol; phenyl methanol; phenyl carbinol; benzenemethanol
Risks
- Combustible.
- Skin contact causes irritation.
- ThermoFisher: SDS
- EPA lists benzyl alcohol as hazardous waste due to ignitability; concentrations over 10% must be disposed of appropriately
Physical and Chemical Properties
Miscible with ethanol, ether, chloroform. Slightly soluble in water.
| Composition | C6H5CH2OH |
|---|---|
| CAS | 100-51-6 |
| Melting Point | -15.19 C |
| Density | 1.040-1.050 g/ml |
| Molecular Weight | mol. wt. = 108.1 |
| Refractive Index | 1.5385-1.5405 |
| Boiling Point | 204.7 C |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 97
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- Richard C. Wolbers, Nanette T. Sterman, Chris Stavroudis, Notes for Workshop on New Methods in the Cleaning of Paintings, J.Paul Getty Trust, Los Angeles, 1990
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Conservation Materials Ltd., Catalog
- Teri Hensick, contributed information, 1998