Difference between revisions of "Kornelite"
Jump to navigation
Jump to search
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
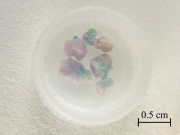
| − | Silky, fibrous pink to violet color crystals of hydrated ferrous sulfate. Kornelite crystals were named after Kornel Hlavacsek, Hungarian geologist. It occurs naturally as a rare, and commercially unimportant, mineral. It generally occurs from the oxidation of iron sulfides. | + | Silky, fibrous pink to violet color crystals of hydrated ferrous sulfate. Kornelite crystals were named after Kornel Hlavacsek, Hungarian geologist. It occurs naturally as a rare, and commercially unimportant, mineral. It generally occurs from the oxidation of iron sulfides ([[pyrite|pyrites]]). |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 20: | Line 20: | ||
==Resources and Citations== | ==Resources and Citations== | ||
* Mindat.org: [https://www.mindat.org/min-2253.html Kornelite] Accessed Dec 2022 | * Mindat.org: [https://www.mindat.org/min-2253.html Kornelite] Accessed Dec 2022 | ||
| − | Web Mineral: [http://webmineral.com/data/Kornelite.shtml Kornelite] Accessed Dec 2022 | + | * Web Mineral: [http://webmineral.com/data/Kornelite.shtml Kornelite] Accessed Dec 2022 |
* Website: http://labs.sci.qut.edu.au/minerals/mineral%20general%20descriptions/K/Kornelitepcd.htm | * Website: http://labs.sci.qut.edu.au/minerals/mineral%20general%20descriptions/K/Kornelitepcd.htm | ||
* Wikipedia: [https://en.wikipedia.org/wiki/Iron(III)_sulfate Iron(III)sulfate] Accessed Dec 2022 | * Wikipedia: [https://en.wikipedia.org/wiki/Iron(III)_sulfate Iron(III)sulfate] Accessed Dec 2022 | ||
Latest revision as of 14:55, 20 December 2022
Description
Silky, fibrous pink to violet color crystals of hydrated ferrous sulfate. Kornelite crystals were named after Kornel Hlavacsek, Hungarian geologist. It occurs naturally as a rare, and commercially unimportant, mineral. It generally occurs from the oxidation of iron sulfides (pyrites).
Synonyms and Related Terms
kornellite (sp); iron (III) sulfate; ferric sulfate heptahydrate
Physical and Chemical Properties
- Composition = Fe2(SO4)3.7H2O
- Soluble in water
- Affected by moisture in air
- Density = 2.306 g/ml
- Monoclinic crystals generally occurring as needles or aggregates
- Cleavage = good in one direction
- Streak = pale red
- Fluorescence = none
Resources and Citations
- Mindat.org: Kornelite Accessed Dec 2022
- Web Mineral: Kornelite Accessed Dec 2022
- Website: http://labs.sci.qut.edu.au/minerals/mineral%20general%20descriptions/K/Kornelitepcd.htm
- Wikipedia: Iron(III)sulfate Accessed Dec 2022