Difference between revisions of "Urea"
(username removed) |
(username removed) |
||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | White, tetragonal prism shaped crystals with an [http://cameo.mfa.org/materials/fullrecord.asp?name=ammonia | + | White, tetragonal prism shaped crystals with an [http://cameo.mfa.org/materials/fullrecord.asp?name=ammonia%20%28anhydrous%29 ammonia] odor. Urea was discovered in 1773 by Hilaire-Marin Rouelle as a constituent of [http://cameo.mfa.org/materials/fullrecord.asp?name=urine urine]. It was first synthesized by Friedrich Wohler in 1828. Urea is used in the manufacture of fertilizers, plastics ([http://cameo.mfa.org/materials/fullrecord.asp?name=polyurethane polyurethanes]), and flameproofing agents. It is also used as a viscosity modifier for [http://cameo.mfa.org/materials/fullrecord.asp?name=starch starch] and [http://cameo.mfa.org/materials/fullrecord.asp?name=casein casein] based paper coatings. Urea is also used by the paper industry to soften [http://cameo.mfa.org/materials/fullrecord.asp?name=cellulose cellulose]. Urea rapidly denatures proteins. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | carbamide; carbonyldiamide; urinstof (Dan.); Harnstoff (Deut.); urea(Esp., It., Sven.); | + | carbamide; carbonyldiamide; urinstof (Dan.); Harnstoff (Deut.); urea(Esp., It., Sven.); urée (Fr.); ureum (Ned.); mocznik (Pol.); uréia (Port.) |
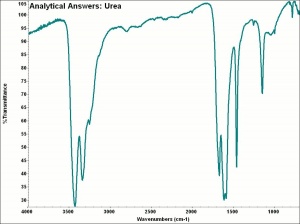
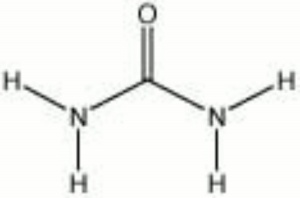
[[[SliderGallery rightalign|aaiUREA.jpg~FTIR|urea.jpg~Chemical structure]]] | [[[SliderGallery rightalign|aaiUREA.jpg~FTIR|urea.jpg~Chemical structure]]] | ||
| Line 40: | Line 40: | ||
== Authority == | == Authority == | ||
| − | * | + | * Palmy Weigle, ''Ancient Dyes for Modern Weavers'', Watson-Guptill Publications, New York, 1974 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 10005 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 10005 | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 836 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
| − | * | + | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 |
* Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Urea (Accessed Nov. 29, 2005) for language synonyms | * Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Urea (Accessed Nov. 29, 2005) for language synonyms | ||
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
Revision as of 07:25, 24 July 2013
Description
White, tetragonal prism shaped crystals with an ammonia odor. Urea was discovered in 1773 by Hilaire-Marin Rouelle as a constituent of urine. It was first synthesized by Friedrich Wohler in 1828. Urea is used in the manufacture of fertilizers, plastics (polyurethanes), and flameproofing agents. It is also used as a viscosity modifier for starch and casein based paper coatings. Urea is also used by the paper industry to soften cellulose. Urea rapidly denatures proteins.
Synonyms and Related Terms
carbamide; carbonyldiamide; urinstof (Dan.); Harnstoff (Deut.); urea(Esp., It., Sven.); urée (Fr.); ureum (Ned.); mocznik (Pol.); uréia (Port.)
Other Properties
Soluble in water, ethanol, benzene. Slightly soluble in ether. Insoluble in chloroform.
| Composition | CO(NH2)2 |
|---|---|
| CAS | 57-13-6 |
| Melting Point | 132.7 |
| Density | 1.335 |
| Molecular Weight | mol. wt. = 60.06 |
Hazards and Safety
Noncombustible.
Mallinckrodt Baker: MSDS
Authority
- Palmy Weigle, Ancient Dyes for Modern Weavers, Watson-Guptill Publications, New York, 1974
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 10005
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 836
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Urea (Accessed Nov. 29, 2005) for language synonyms
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998