Difference between revisions of "Rutile"
(username removed) |
|||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | One of three naturally occurring isomorphic forms of [ | + | One of three naturally occurring isomorphic forms of [[titanium dioxide]]: [[anatase]], rutile, and [[brookite]]. Rutile forms a hard, reddish brown crystal with a metallic luster. Small amounts of natural rutile may have been used as a pale yellow to brown pigment. Synthetic rutile is produced both as a gemstone crystal and as a white powder for paint. Rutile gemstone crystals prepared by the Verneuil process are used as imitation diamonds in paste jewelry. The anatase form of titanium dioxide was a common white pigment in the early 20th century. Once the first commercially viable method for producing rutile was developed in 1938, production shifted to rutile because white paints with anatase pigments were subject to chalking and yellowing As a pigment, rutile has good opacity, a high refractive index and is nontoxic. It is the most important white pigment in the commercial paint industry even though its color tends to be slightly yellow. |
[[File:Gemrutilekes.jpg|thumb|Rutilated quartz pendant]] | [[File:Gemrutilekes.jpg|thumb|Rutilated quartz pendant]] | ||
| + | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Revision as of 13:18, 31 July 2014
Description
One of three naturally occurring isomorphic forms of Titanium dioxide: Anatase, rutile, and Brookite. Rutile forms a hard, reddish brown crystal with a metallic luster. Small amounts of natural rutile may have been used as a pale yellow to brown pigment. Synthetic rutile is produced both as a gemstone crystal and as a white powder for paint. Rutile gemstone crystals prepared by the Verneuil process are used as imitation diamonds in paste jewelry. The anatase form of titanium dioxide was a common white pigment in the early 20th century. Once the first commercially viable method for producing rutile was developed in 1938, production shifted to rutile because white paints with anatase pigments were subject to chalking and yellowing As a pigment, rutile has good opacity, a high refractive index and is nontoxic. It is the most important white pigment in the commercial paint industry even though its color tends to be slightly yellow.
Synonyms and Related Terms
titanium dioxide; synthetic rutile; imitation diamond; rutile (Eng., Fr.); rutilo (It., Esp., Port.); rutil (Nor., Sven.); ; Titanweiss (Rutil) (Deut.); leyko toy titanioy (Roytilio) (Gr.); bianco di titanio (rutilo) (It.); titaandioxide (rutiel) (Ned.); rútilo (Port.)
Other Properties
Insoluble in acids. Tetragonal crystal system with prismatic habit (often twinned). Crystals may appear red in transparent light. Particle size 0.2 - 0.5 micrometers
High birefringence and interference colors Fluoresces gray or dark purple
| Composition | TiO2 |
|---|---|
| Mohs Hardness | 6.0 - 6.5 |
| Melting Point | 1640 |
| Density | 3.75-4.3 |
| Refractive Index | 2.71 - 2.72 |
Hazards and Safety
Nontoxic. No significant hazards.
Additional Information
° M.Laver, "Titanium Dioxide Whites", Artists Pigments, Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997. ° Mineralogy Database: Rutile° Walter C. McCrone, "Polarized Light Microscopy in Conservation: A Personal Perspective" JAIC 33(2):101-14, 1994. (contains a table of dates on the history of titanium white as a pigment)
Comparisons
Natural and Simulated Diamonds
Characteristics of Common White Pigments
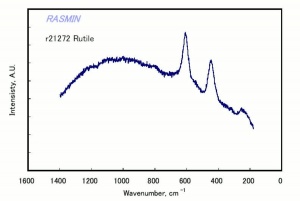
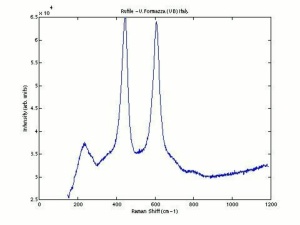
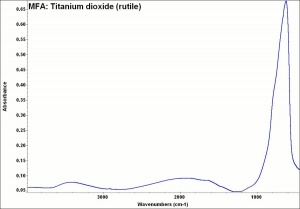
Additional Images
Authority
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density=4.2 and ref.index.=2.9, 2.6
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 815
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Encyclopedia Britannica, http://www.britannica.com Comment: "rutile" Encyclopædia Britannica [Accessed January 22, 2002].
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980 Comment: ref. index = 2.61; 1.90;
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Rutile (Accessed Sept. 14, 2005)
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998