Difference between revisions of "Limewater"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 32: | Line 32: | ||
[http://www.cdc.gov/niosh/ipcsneng/neng0408.html International Chemical Safety Card] | [http://www.cdc.gov/niosh/ipcsneng/neng0408.html International Chemical Safety Card] | ||
| − | == | + | == Sources Checked for Data in Record == |
* Thomas Gregory, ''The Condensed Chemical Dictionary'', Reinhold Publishing, New York, 3rd ed., 1942 | * Thomas Gregory, ''The Condensed Chemical Dictionary'', Reinhold Publishing, New York, 3rd ed., 1942 | ||
Revision as of 06:30, 1 May 2016
Description
An aqueous solution of Calcium hydroxide produced by slaking Lime. Limewater, is a colorless somewhat milky solution that is strongly alkaline even though calcium hydroxide is only slightly soluble in water. Limewater was used to saturate plaster before the application of secco colors. It is also used in Calamine solutions and as an antacid.
Synonyms and Related Terms
calcium hydroxide; milk of lime; lime water
Other Properties
A saturated solution of calcium hydroxide has a pH of 12.4.
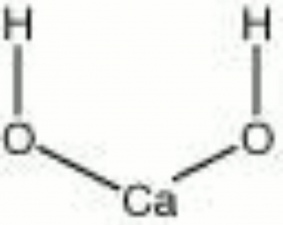
| Composition | Ca(OH)2 |
|---|---|
| CAS | 1305-62-0 |
| Melting Point | 580 (dec) |
| Molecular Weight | mol. wt. = 74.1 |
Hazards and Safety
International Chemical Safety Card
Sources Checked for Data in Record
- Thomas Gregory, The Condensed Chemical Dictionary, Reinhold Publishing, New York, 3rd ed., 1942
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- External source or communication Comment: Eric Hansen, contributed information (April 2001)
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985