Difference between revisions of "Magnesium carbonate"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 51: | Line 51: | ||
| − | == | + | == Sources Checked for Data in Record == |
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | ||
Revision as of 06:46, 1 May 2016
Description
A fluffy white powder used as an inert pigment and as an ingredient in inks, glass, ceramic glazes, and dentifrice. Magnesium carbonate has also been used as a sorbent powder for water-free (dry) cleaning of jewelry and doll hair. Aqueous solutions of magnesium carbonate are used for neutralization and alkalization of paper.
See also Magnesium bicarbonate).
Synonyms and Related Terms
magnesite; magnesia white; Pigment White 18; Magnesiumcarbonat (Deut.)
Other Properties
Soluble in acids. Slightly soluble in water. Insoluble in ethanol.
Translucent, colorless, angular crystals; high birefringence under crossed polars; extinction is complete and straight.
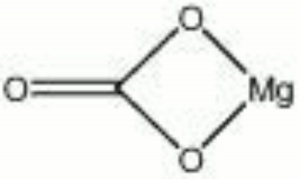
| Composition | MgCO3 |
|---|---|
| CAS | 546-93-0 |
| Melting Point | 350 (dec) |
| Density | 3.0 |
| Molecular Weight | mol. wt. = 84.3 |
| Refractive Index | 1.508; 1.510; 1.700 |
Hazards and Safety
Nontoxic. Ingestion has a laxative effect. Noncombustible.
LINK: International Chemical Safety Card
Comparisons
Characteristics of Common White Pigments
Sources Checked for Data in Record
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Tom Rowland, Noel Riley, A-Z Guide to Cleaning, Conserving and Repairing Antiques, Constable and Co., Ltd., London, 1981
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5696