Difference between revisions of "Anatase"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | One of three naturally occuring isomorphic forms of [[titanium%20dioxide|titanium dioxide]]: anatase, rutile, and brookite. Anatase forms | + | One of three naturally occuring isomorphic forms of [[titanium%20dioxide|titanium dioxide]]: anatase, rutile, and brookite. Anatase forms translucent or transparent crystals (octahedrite) varying in color from black to reddish brown, yellowish brown, dark blue or gray. Deposits have been found in the Alps, Brazil, and the Ural Mountains; it is also formed by the weathering of titanite (sphene) and ilmenite. Mineral anatase is not a major component of artists' materials; however, it has been reported as a minor constituent (<2%)in white paints on ancient artifacts from diverse sites, as a mineral impurity in white clay-based pigments. |
| + | |||
| + | Anatase was produced synthetically in the early 20th century for use as a white paint pigment with remarkable hiding power. The most successful processes were based on extraction from crushed and roasted ilmenite (iron titanate). The major problems in producing a white anatase pigment were to remove iron, a major element in the ilmenite starting material, and to prevent the formation of rutile during the final calcination step; the early experimental pigments typically were not bright white as they contained impurities such as residual iron as well as some rutile. For at least a decade from about 1908 onward, laboratory research into possible purification and production methods was being conducted in Europe and the United States. Although the first commercial product was a less than pure pigment (the Prima X line, 83% TiO2) produced in Norway (Titan Company A/S) in 1918 to 1919, this was almost immediately replaced by a composite pigment containing calcium phosphate and barium sulfate in 1919; the early composites were described as the color of “old India ivory.” In the United States (Titanium Pigment Company), pure pigment was only produced in small experimental batches until 1926 (Titanox A, 98% TiO2), and the pigments which were commercially available from 1916 until 1925 were composites with barium sulphate. The Americans eventually adopted the Norwegian technology and the two companies amalgamated in 1920. In France, a process was discovered in 1920 to allow production of pure anatase titanium dioxide pigment, but only in 1923 did production of a 96-99% titanium dioxide pigment begin; the pure anatase had significantly greater hiding power than the composities. Pigment purity continued to be a problem, however, and even by 1927 the pure anatase was described as slightly yellow. Because rutile had advantageous weathering properties (less chalking) and higher hiding power (up to 25% improvement over pure anatase), research to improve experimental rutile-based pigments that were patented in 1931 culminated in commercial production in Europe and in the United States in 1937. | ||
| + | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | octahedrite; titanium dioxide; anatase (Eng., Fr., Port., Nor.); anastase (sp); Anatas (Deut.); anatasio (It.); anatasa (Esp.); anataas (Ned.) | + | octahedrite; titanium dioxide; anatase (Eng., Fr., Port., Nor.); anastase (sp); Anatas (Deut.); anatasio (It.); anatasa (Esp.); anataas (Ned.) |
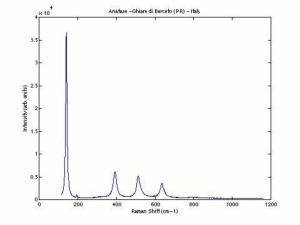
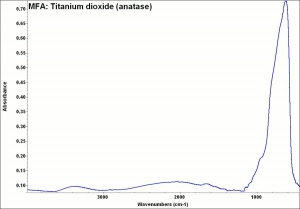
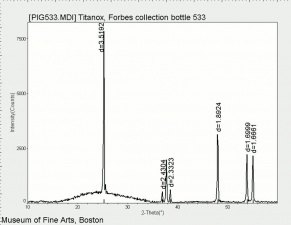
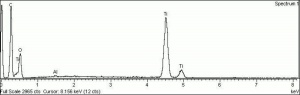
[[[SliderGallery rightalign|Anataseitaly1.jpg~Raman|MFA- Titanium dioxide (anatase).jpg~FTIR|PIG533.jpg~XRD|f533sem.jpg~SEM|f533edsbw.jpg~EDS]]] | [[[SliderGallery rightalign|Anataseitaly1.jpg~Raman|MFA- Titanium dioxide (anatase).jpg~FTIR|PIG533.jpg~XRD|f533sem.jpg~SEM|f533edsbw.jpg~EDS]]] | ||
| Line 12: | Line 15: | ||
== Other Properties == | == Other Properties == | ||
| − | Tetragonal crystal system. Particle size 0.2 - 0.5 micrometers. | + | Tetragonal crystal system. |
| + | |||
| + | Optic sign uniaxial, negative. | ||
| + | |||
| + | Particle size of modern pigment 0.2 - 0.5 micrometers. | ||
| + | |||
| + | High birefringence under crossed polars. | ||
| + | |||
| + | Non-fluorescent to weak white fluorescence (pigment). | ||
| − | At high temperatures anatase will convert to rutile | + | At high temperatures (400-1200C) anatase will convert to rutile. |
| − | |||
{| class="wikitable" | {| class="wikitable" | ||
| Line 27: | Line 37: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | | + | | transforms to rutile |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 3. | + | | 3.7 (coated pigment)- 3.9 |
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| − | | 2.54 - 2.55 | + | | 2.54 - 2.55 (mineral, pure pigment) |
| + | | 1.87 - 2.3 (composite pigment) | ||
|} | |} | ||
| Line 40: | Line 51: | ||
Nontoxic. No significant hazards. | Nontoxic. No significant hazards. | ||
| − | + | Chemically inert, insoluble in water, organic solvents, aqueous alkalis; can be dissolved in sulfuric or hydrofluoric acid; slow to dry in oil without additives or surface treatment. | |
| + | |||
| + | Strong UV absorber, photochemically active and can cause deterioration of admixed media if exposed to ultraviolet light; early anatase pigments showed considerable tendency to chalk on exposure and could discolor. | ||
== Additional Information == | == Additional Information == | ||
| Line 75: | Line 88: | ||
* Website address 1 Comment: Pigments Through the Ages at http://webexhibits.org/pigments : Refractive index: anatase: 2.3 - 2.65 | * Website address 1 Comment: Pigments Through the Ages at http://webexhibits.org/pigments : Refractive index: anatase: 2.3 - 2.65 | ||
| + | |||
| + | * Buzgar et al., "The Raman Study of the White Pigment used in Cucuteni Pottery," Analele Stiintifice ale Universitatii “Al. I. Cuza” din Iasi Seria Geologie 59(2) 2013) 41–50; also http://geology.uaic.ro/auig/articole/2013%20no2/1_L03-Buzgar.pdf | ||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 17:21, 20 November 2016
Description
One of three naturally occuring isomorphic forms of Titanium dioxide: anatase, rutile, and brookite. Anatase forms translucent or transparent crystals (octahedrite) varying in color from black to reddish brown, yellowish brown, dark blue or gray. Deposits have been found in the Alps, Brazil, and the Ural Mountains; it is also formed by the weathering of titanite (sphene) and ilmenite. Mineral anatase is not a major component of artists' materials; however, it has been reported as a minor constituent (<2%)in white paints on ancient artifacts from diverse sites, as a mineral impurity in white clay-based pigments.
Anatase was produced synthetically in the early 20th century for use as a white paint pigment with remarkable hiding power. The most successful processes were based on extraction from crushed and roasted ilmenite (iron titanate). The major problems in producing a white anatase pigment were to remove iron, a major element in the ilmenite starting material, and to prevent the formation of rutile during the final calcination step; the early experimental pigments typically were not bright white as they contained impurities such as residual iron as well as some rutile. For at least a decade from about 1908 onward, laboratory research into possible purification and production methods was being conducted in Europe and the United States. Although the first commercial product was a less than pure pigment (the Prima X line, 83% TiO2) produced in Norway (Titan Company A/S) in 1918 to 1919, this was almost immediately replaced by a composite pigment containing calcium phosphate and barium sulfate in 1919; the early composites were described as the color of “old India ivory.” In the United States (Titanium Pigment Company), pure pigment was only produced in small experimental batches until 1926 (Titanox A, 98% TiO2), and the pigments which were commercially available from 1916 until 1925 were composites with barium sulphate. The Americans eventually adopted the Norwegian technology and the two companies amalgamated in 1920. In France, a process was discovered in 1920 to allow production of pure anatase titanium dioxide pigment, but only in 1923 did production of a 96-99% titanium dioxide pigment begin; the pure anatase had significantly greater hiding power than the composities. Pigment purity continued to be a problem, however, and even by 1927 the pure anatase was described as slightly yellow. Because rutile had advantageous weathering properties (less chalking) and higher hiding power (up to 25% improvement over pure anatase), research to improve experimental rutile-based pigments that were patented in 1931 culminated in commercial production in Europe and in the United States in 1937.
Synonyms and Related Terms
octahedrite; titanium dioxide; anatase (Eng., Fr., Port., Nor.); anastase (sp); Anatas (Deut.); anatasio (It.); anatasa (Esp.); anataas (Ned.)
Other Properties
Tetragonal crystal system.
Optic sign uniaxial, negative.
Particle size of modern pigment 0.2 - 0.5 micrometers.
High birefringence under crossed polars.
Non-fluorescent to weak white fluorescence (pigment).
At high temperatures (400-1200C) anatase will convert to rutile.
| Composition | TiO2 | |
|---|---|---|
| Mohs Hardness | 5.0 - 6.0 | |
| Melting Point | transforms to rutile | |
| Density | 3.7 (coated pigment)- 3.9 | |
| Refractive Index | 2.54 - 2.55 (mineral, pure pigment) | 1.87 - 2.3 (composite pigment) |
Hazards and Safety
Nontoxic. No significant hazards.
Chemically inert, insoluble in water, organic solvents, aqueous alkalis; can be dissolved in sulfuric or hydrofluoric acid; slow to dry in oil without additives or surface treatment.
Strong UV absorber, photochemically active and can cause deterioration of admixed media if exposed to ultraviolet light; early anatase pigments showed considerable tendency to chalk on exposure and could discolor.
Additional Information
° M.Laver, "Titanium Dioxide Whites", Artists Pigments, Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997.
° Walter C. McCrone, "Polarized Light Microscopy in Conservation: A Personal Perspective" JAIC 33(2):101-14, 1994. (contains a table of dates on the history of titanium white as a pigment)
Comparisons
Characteristics of Common White Pigments
Sources Checked for Data in Record
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 815
- Encyclopedia Britannica, http://www.britannica.com Comment: "anatase" Encyclopædia Britannica [Accessed January 22, 2002].;
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: Ref. Index = 2.5; density = 3.9
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980 Comment: Ref. index = 2.56, 2.49
- Artists' Pigments: A Handbook of their History and Characteristics, Elisabeth West FitzHugh, Oxford University Press, Oxford, Vol. 3, 1997 Comment: M.Laver, "Titanium Dioxide Whites"
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Anatase
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Website address 1 Comment: Pigments Through the Ages at http://webexhibits.org/pigments : Refractive index: anatase: 2.3 - 2.65
- Buzgar et al., "The Raman Study of the White Pigment used in Cucuteni Pottery," Analele Stiintifice ale Universitatii “Al. I. Cuza” din Iasi Seria Geologie 59(2) 2013) 41–50; also http://geology.uaic.ro/auig/articole/2013%20no2/1_L03-Buzgar.pdf