Difference between revisions of "Camphene"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 2: | Line 2: | ||
A colorless, crystalline material. Camphene is a terpene type compound obtained from [[camphor|camphor oil]] or synthesized from [[turpentine (oil)|turpentine]]. Camphene was used as a camphor substitute and as an [[insecticide]]. The name camphene has been mistakenly used as a synonym for [[burning fluid]], which is a 19th century commercial lamp oil containing a turpentine and ethanol mixture that burned brightly but was potentially explosive. | A colorless, crystalline material. Camphene is a terpene type compound obtained from [[camphor|camphor oil]] or synthesized from [[turpentine (oil)|turpentine]]. Camphene was used as a camphor substitute and as an [[insecticide]]. The name camphene has been mistakenly used as a synonym for [[burning fluid]], which is a 19th century commercial lamp oil containing a turpentine and ethanol mixture that burned brightly but was potentially explosive. | ||
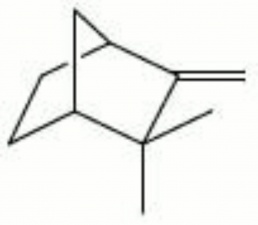
| − | + | [[[SliderGallery rightalign|camphene.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
2,2-dimethyl-3-methylenebycyclo-[2,2,2]heptane | 2,2-dimethyl-3-methylenebycyclo-[2,2,2]heptane | ||
| + | == Risks == | ||
| − | [ | + | * Toxic by ingestion. |
| + | * Flammable solid. Flash point = 36 C. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/83984.htm MSDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
| − | Soluble in ether, cyclohexane, cyclohexene, dioxane, chloroform. Slightly soluble in ethanol. | + | Soluble in ether, cyclohexane, cyclohexene, dioxane, chloroform. Slightly soluble in ethanol. Insoluble in water. Cubic crystals. |
{| class="wikitable" | {| class="wikitable" | ||
| Line 22: | Line 25: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 51-52 | + | | 51-52 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.8422 | + | | 0.8422 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 37: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 158.5-159.5 | + | | 158.5-159.5 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * Charles Leib, August 2008, Submitted information. |
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 832 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 832 | ||
Latest revision as of 14:44, 18 May 2022
Description
A colorless, crystalline material. Camphene is a terpene type compound obtained from camphor oil or synthesized from turpentine. Camphene was used as a camphor substitute and as an Insecticide. The name camphene has been mistakenly used as a synonym for Burning fluid, which is a 19th century commercial lamp oil containing a turpentine and ethanol mixture that burned brightly but was potentially explosive.
Synonyms and Related Terms
2,2-dimethyl-3-methylenebycyclo-[2,2,2]heptane
Risks
- Toxic by ingestion.
- Flammable solid. Flash point = 36 C.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in ether, cyclohexane, cyclohexene, dioxane, chloroform. Slightly soluble in ethanol. Insoluble in water. Cubic crystals.
| Composition | C10H16 |
|---|---|
| CAS | 79-92-5 |
| Melting Point | 51-52 C |
| Density | 0.8422 g/ml |
| Molecular Weight | mol. wt. = 136.24 |
| Refractive Index | 1.45514 |
| Boiling Point | 158.5-159.5 C |
Resources and Citations
- Charles Leib, August 2008, Submitted information.
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 832
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1777
- MSDS Sheet Comment: Fisher Scientific 8/20/02: mp = 36.00 - 38.00 deg C
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998