Difference between revisions of "Paratoluidine"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | Transparent colorless crystals prepared by treating nitrotoluene with [ | + | Transparent colorless crystals prepared by treating nitrotoluene with [[acetic%20acid|acetic acid]] in the presence of [[iron|iron]]. Paratoluidine is used in the manufacture of some synthetic organic red colorants. Paratoluidine reds are sometimes used in inks but are not used as an artist colors because they bleed and fade with time. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Revision as of 11:07, 10 May 2016
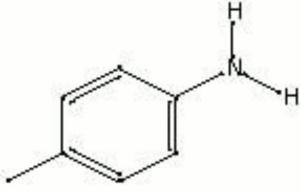
Description
Transparent colorless crystals prepared by treating nitrotoluene with Acetic acid in the presence of Iron. Paratoluidine is used in the manufacture of some synthetic organic red colorants. Paratoluidine reds are sometimes used in inks but are not used as an artist colors because they bleed and fade with time.
Synonyms and Related Terms
p-toluidine; 4-aminotoluene; 4-amino-1-methylbenzene; p-methylaniline
Other Properties
Soluble in alcohols, ether, acetone, carbon disulfide, oils, dilute acids. Slightly soluble in water.
Darkens on exposure to air.
| Composition | C6H4CH3NH2 |
|---|---|
| CAS | 106-49-0 |
| Melting Point | 44-45 |
| Density | 1.046 |
| Molecular Weight | mol. wt. = 107.2 |
| Boiling Point | 200-201 |
Hazards and Safety
Toxic by ingestion, inhalation and skin absorption.
Combustible. Flash point = 86C (188F)
International Chemical Safety Card
Sources Checked for Data in Record
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9396
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9366
- F. Crace-Calvert, Dyeing and Calico Printing, Palmer & Howe, London, 1876