Difference between revisions of "Nitroglycerin"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A heavy, poisonous, oily compound that is unstable and can readily explode. Nitroglycerin was discovered by Ascanio Sobrero in 1847. A safe manufacturing process was developed by Alfred Nobel in the 1860s. Nitroglycerin is prepared by the nitration of [[glycerol | + | A heavy, poisonous, oily compound that is unstable and can readily explode. Nitroglycerin was discovered by Ascanio Sobrero in 1847. A safe manufacturing process was developed by Alfred Nobel in the 1860s. Nitroglycerin is prepared by the nitration of [[glycerol]]. It is used to make dynamite. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
nitroglycerine; glyceryl trinitrate; trinitroglycerin; Swedish blasting oil; nitroglycerin (Dan., Sven.); Glycerintrinitrat (Deut.); nitroglicerina (Esp., It., Port.); nitroglycérine (Fr.); trinitrine (Fr.); Nitroglycerine (Ned.); nitrogliceryna (Pol.); triazotan glicerol (Pol.); (Port.) | nitroglycerine; glyceryl trinitrate; trinitroglycerin; Swedish blasting oil; nitroglycerin (Dan., Sven.); Glycerintrinitrat (Deut.); nitroglicerina (Esp., It., Port.); nitroglycérine (Fr.); trinitrine (Fr.); Nitroglycerine (Ned.); nitrogliceryna (Pol.); triazotan glicerol (Pol.); (Port.) | ||
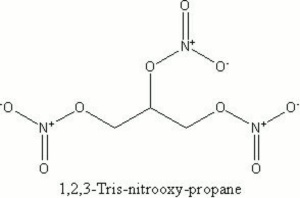
| + | [[[SliderGallery rightalign|nitroglycerinvt.jpg~Chemical structure]]] | ||
| + | == Risks == | ||
| + | * Compound can be desensitized by cooling to 5-10 C but then should never be warmed as thawing is extremely hazardous. | ||
| + | * Pfizer: [https://cdn.pfizer.com/pfizercom/products/material_safety_data/NITROSTAT_(nitroglycerin)_Tablets_(0.4_0.6mg)6-may-2019.pdf SDS] | ||
| + | ==Physical and Chemical Properties== | ||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
| Line 16: | Line 21: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 13.2 | + | | 13.2 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.13 | + | | 1.13 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 25: | Line 30: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 50-60 (decomposes) | + | | 50-60 C (decomposes) |
|} | |} | ||
| − | + | ==Resources and Citations== | |
| − | |||
| − | == | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * Art and Architecture Thesaurus Online, | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 |
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Nitroglycerin (Accessed Oct. 18, 2005) |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 11:12, 19 October 2022
Description
A heavy, poisonous, oily compound that is unstable and can readily explode. Nitroglycerin was discovered by Ascanio Sobrero in 1847. A safe manufacturing process was developed by Alfred Nobel in the 1860s. Nitroglycerin is prepared by the nitration of Glycerol. It is used to make dynamite.
Synonyms and Related Terms
nitroglycerine; glyceryl trinitrate; trinitroglycerin; Swedish blasting oil; nitroglycerin (Dan., Sven.); Glycerintrinitrat (Deut.); nitroglicerina (Esp., It., Port.); nitroglycérine (Fr.); trinitrine (Fr.); Nitroglycerine (Ned.); nitrogliceryna (Pol.); triazotan glicerol (Pol.); (Port.)
Risks
- Compound can be desensitized by cooling to 5-10 C but then should never be warmed as thawing is extremely hazardous.
- Pfizer: SDS
Physical and Chemical Properties
| Composition | C3H5N3O9 |
|---|---|
| CAS | 55-63-0 |
| Melting Point | 13.2 C |
| Density | 1.13 g/ml |
| Molecular Weight | 227.0872 |
| Boiling Point | 50-60 C (decomposes) |
Resources and Citations
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Wikipedia: http://en.wikipedia.org/wiki/Nitroglycerin (Accessed Oct. 18, 2005)