Difference between revisions of "Phenylmercuric chloride"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| Line 6: | Line 6: | ||
chlorophenylmercury; phenyl mercuric chloride; Stopspot; (chloromercuri)benzene; mercuriphenyl chloride; phenyl chloromercury | chlorophenylmercury; phenyl mercuric chloride; Stopspot; (chloromercuri)benzene; mercuriphenyl chloride; phenyl chloromercury | ||
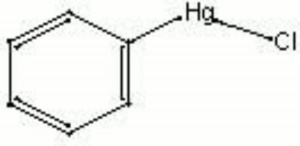
| + | [[[SliderGallery rightalign|phenylmercuric chloride.jpg~Chemical structure]]] | ||
| − | + | == Risks == | |
| − | == | + | * Highly toxic by ingestion, inhalation and skin absorption. |
| + | * Causes burns and nerve disorders. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/98987.htm MSDS] | ||
| + | == Physical and Chemical Properties == | ||
Soluble in benzene, ether, pyridine. Slightly soluble in hot ethanol. Insoluble in water. | Soluble in benzene, ether, pyridine. Slightly soluble in hot ethanol. Insoluble in water. | ||
| Line 22: | Line 26: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 250-252 | + | | 250-252 C |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 28: | Line 32: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 07:37, 22 October 2022
Description
White satiny crystals that have been used as a Disinfectant, and Fungicide. Phenylmercuric chloride is no longer recommended for use due to the hazards of Mercury compounds to humans and the environment.
Synonyms and Related Terms
chlorophenylmercury; phenyl mercuric chloride; Stopspot; (chloromercuri)benzene; mercuriphenyl chloride; phenyl chloromercury
Risks
- Highly toxic by ingestion, inhalation and skin absorption.
- Causes burns and nerve disorders.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in benzene, ether, pyridine. Slightly soluble in hot ethanol. Insoluble in water.
| Composition | C6H5HgCl |
|---|---|
| CAS | 100-56-1 |
| Melting Point | 250-252 C |
| Molecular Weight | mol. wt. = 313.14 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 7182