Difference between revisions of "Phthalic acid"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| Line 9: | Line 9: | ||
[[[SliderGallery rightalign|phthalic acid.jpg~Chemical structure]]] | [[[SliderGallery rightalign|phthalic acid.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Irritating to mucous membranes and skin. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC131072500&productDescription=PHTHALIC+ACID%2C+99%25+250GRPHTHA&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| + | ==Physical and Chemical Properties== | ||
Soluble in ethanol. Slightly soluble in water, ether. Insoluble in chloroform. | Soluble in ethanol. Slightly soluble in water, ether. Insoluble in chloroform. | ||
| Line 22: | Line 26: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 191-210 (dec) | + | | 191-210 C (dec) |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.585-1.593 | + | | 1.585-1.593 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 35: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 45: | Line 43: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 7527 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 7527 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Phthalic_acid (accessed Mar. 14, 2006) |
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
Revision as of 11:18, 4 August 2022
Description
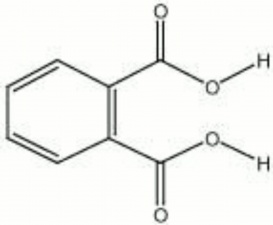
Colorless, odorless crystalline Organic acid. Phthalic acid was first synthesized by Auguste Laurent in 1836. It is used in the manufacture of several dyes including Indigo and Phenolphthalein.
Synonyms and Related Terms
o-phthalic acid; o-benzene dicarboxylic acid; o-dicarboxybenzene
Risks
- Irritating to mucous membranes and skin.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in ethanol. Slightly soluble in water, ether. Insoluble in chloroform.
| Composition | C6H4(COOH)2 |
|---|---|
| CAS | 88-99-3 |
| Melting Point | 191-210 C (dec) |
| Density | 1.585-1.593 g/ml |
| Molecular Weight | mol. wt.=166.14 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Hoechst Celanese Corporation, Dictionary of Fiber & Textile Technology (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 7527
- Wikipedia: http://en.wikipedia.org/wiki/Phthalic_acid (accessed Mar. 14, 2006)
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998