Difference between revisions of "Sodium phosphate, dibasic"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| Line 9: | Line 9: | ||
[[[SliderGallery rightalign|sodium phosphate, dibasic.jpg~Chemical structure]]] | [[[SliderGallery rightalign|sodium phosphate, dibasic.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Nonflammable. | ||
| + | * Thermo Fisher; {https://www.fishersci.com/store/msds?partNumber=AC124992500&productDescription=SODIUM+PHOSPHATE+DIBASIC+250GR&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
Soluble in water, ethanol. | Soluble in water, ethanol. | ||
| Line 22: | Line 27: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 240 | + | | 240 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.07 | + | | 2.07 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 39: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 09:08, 2 June 2022
Description
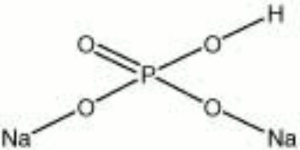
White, crystalline, hygroscopic powder. Dibasic sodium phosphate, or DSP, occurs in several hydrated forms from anhydrous to dodecahydrate. It is used in the manufacture of dyes, fertilizers, detergents, ceramics, and enamels. DSP is used as a Sequestrant, Emulsifier, and fire retardant. It acts as a Mordant in dyeing and is used for weighting Silk. DSP is also used as a replacement for Borax in soldering and brazing. In addition, DSP is used in bleach baths for color photographs.
Synonyms and Related Terms
DSP; disodium phosphate; secondary sodium orthophosphate; disodium hydrogen phosphate; phosphate of soda; Sorensen's phosphate (dihydrate)
Risks
- Nonflammable.
- Thermo Fisher; {https://www.fishersci.com/store/msds?partNumber=AC124992500&productDescription=SODIUM+PHOSPHATE+DIBASIC+250GR&vendorId=VN00032119&countryCode=US&language=en SDS]
Physical and Chemical Properties
Soluble in water, ethanol.
| Composition | Na2HPO4 - xH2O |
|---|---|
| CAS | 7558-79-4 |
| Melting Point | 240 C |
| Density | 2.07 g/ml |
| Molecular Weight | mol. wt. = 141.96 |
| Refractive Index | 1.4412, 1.4424, 1.4526 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Thomas C. Jester (ed.), Twentieth-Century Building Materials, McGraw-Hill Companies, Washington DC, 1995
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8805
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index = 1.4412, 1.4424, 1.4526