Difference between revisions of "Marble"
| Line 1: | Line 1: | ||
| − | [[File:mMarble dog.jpg|thumb|]] | + | [[File:mMarble dog.jpg|thumb|'Arno' sculpture by Greenought<br>MFA# 1973.601]] |
== Description == | == Description == | ||
A hard, dense, crystalline stone primarily composed of [[calcium%20carbonate|calcium carbonate]]. Marble is [[limestone|limestone]] or [[dolomite|dolomite]] that has been metamorphosed with heat and pressure. Pure calcite marble is white, but impurities produce a wide variety of coloring and patterns. Marble has fine grains and polishes to a smooth, high gloss. It is used for statuary and buildings. Marble has been quarried from sites around the world since at least the seventh century BCE. Historical quarry locations and marble types include: | A hard, dense, crystalline stone primarily composed of [[calcium%20carbonate|calcium carbonate]]. Marble is [[limestone|limestone]] or [[dolomite|dolomite]] that has been metamorphosed with heat and pressure. Pure calcite marble is white, but impurities produce a wide variety of coloring and patterns. Marble has fine grains and polishes to a smooth, high gloss. It is used for statuary and buildings. Marble has been quarried from sites around the world since at least the seventh century BCE. Historical quarry locations and marble types include: | ||
| − | + | [[File:2002.630-SC53335.jpg|thumb|Marble relief<br>MFA# 2002.630]] | |
- Greece: Paros (Parian marble), Penteli (Pentelic marble), rosso antico, Eleusinian marble | - Greece: Paros (Parian marble), Penteli (Pentelic marble), rosso antico, Eleusinian marble | ||
| Line 19: | Line 19: | ||
Note: Commercially, the term 'marble' is used for any non-granite stone that can take a polish, such as travertine, onyx, serpentine, and limestone. | Note: Commercially, the term 'marble' is used for any non-granite stone that can take a polish, such as travertine, onyx, serpentine, and limestone. | ||
| − | |||
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | + | calcium carbonate; marmer (Ned.); Marmor (Deut., Sven.); marbre (Fr.); marmo (It.); mármol (Esp.); marmur (Pol.); mármore (Port.); | |
| + | ==Physical and Chemical Properties== | ||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
| Line 36: | Line 35: | ||
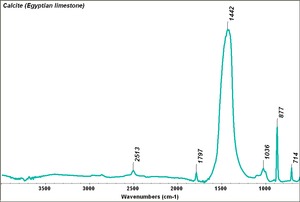
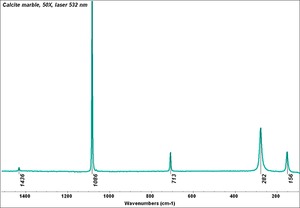
[[[SliderGallery rightalign|Calcite (Egyptian limestone).TIF~FTIR (MFA)||Calcite marble, 50X, laser 532 nm copy.tif~Raman (MFA)]]] | [[[SliderGallery rightalign|Calcite (Egyptian limestone).TIF~FTIR (MFA)||Calcite marble, 50X, laser 532 nm copy.tif~Raman (MFA)]]] | ||
| − | |||
| − | |||
| − | |||
| − | |||
== Additional Images == | == Additional Images == | ||
| Line 48: | Line 43: | ||
</gallery> | </gallery> | ||
| − | + | == Resources and Citations == | |
| − | == | ||
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| Line 59: | Line 53: | ||
* Thomas C. Jester (ed.), ''Twentieth-Century Building Materials'', McGraw-Hill Companies, Washington DC, 1995 | * Thomas C. Jester (ed.), ''Twentieth-Century Building Materials'', McGraw-Hill Companies, Washington DC, 1995 | ||
| − | * Website | + | * Website: http://www.marble-institute.com |
* Janet Burnett Grossman, ''Looking at Greek and Roman Sculpture in Stone'', J. Paul Getty Trust, Los Angeles, 2003 | * Janet Burnett Grossman, ''Looking at Greek and Roman Sculpture in Stone'', J. Paul Getty Trust, Los Angeles, 2003 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Marble (Accessed Nov. 9, 2005) |
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
Revision as of 06:13, 11 August 2020
Description
A hard, dense, crystalline stone primarily composed of Calcium carbonate. Marble is Limestone or Dolomite that has been metamorphosed with heat and pressure. Pure calcite marble is white, but impurities produce a wide variety of coloring and patterns. Marble has fine grains and polishes to a smooth, high gloss. It is used for statuary and buildings. Marble has been quarried from sites around the world since at least the seventh century BCE. Historical quarry locations and marble types include:
- Greece: Paros (Parian marble), Penteli (Pentelic marble), rosso antico, Eleusinian marble
- Turkey: Proconnesus
- Italy: Carrara, bardiglio, Cipollino marble (cipolin), parmazo marble
- Spain: Macael
- Belgium: rance, Belgian black, St. Anne marble
- France: Languedoc marble, griotte, Sarrancolin marble
- U.S.: Vermont white statuary, Georgia white, Colorado Yule statuary, Alabama cream, Tennessee pink, Rockingham royal black.
Note: Commercially, the term 'marble' is used for any non-granite stone that can take a polish, such as travertine, onyx, serpentine, and limestone.
Synonyms and Related Terms
calcium carbonate; marmer (Ned.); Marmor (Deut., Sven.); marbre (Fr.); marmo (It.); mármol (Esp.); marmur (Pol.); mármore (Port.);
Physical and Chemical Properties
| Mohs Hardness | 3.0 |
|---|---|
| Density | 2.6-2.84 |
Additional Images
Resources and Citations
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Thomas C. Jester (ed.), Twentieth-Century Building Materials, McGraw-Hill Companies, Washington DC, 1995
- Website: http://www.marble-institute.com
- Janet Burnett Grossman, Looking at Greek and Roman Sculpture in Stone, J. Paul Getty Trust, Los Angeles, 2003
- Wikipedia: http://en.wikipedia.org/wiki/Marble (Accessed Nov. 9, 2005)
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=2.6-2.84 (160-177 pounds per cubic foot)