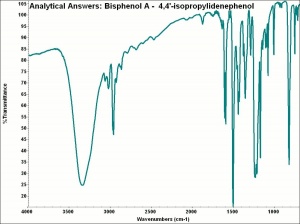
Difference between revisions of "Bisphenol A"
Jump to navigation
Jump to search
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | White flaky material with a phenolic odor. Bisphenol A is one of the primary components in many [[epoxy]] resins. It is also used to make [[polycarbonate| | + | White flaky material with a phenolic odor. Bisphenol A (BPA) is one of the primary components in many [[epoxy]] resins. It is also used to make [[polycarbonate|Polycarbonates]], [[phenoxy%20resin|Phenoxy resins]], [[Polysulfone]] resins as well as some [[flame%20retardant|Flame retardants]] and [[fungicide|Fungicides]]. The plastic resins containing BPA are used in consumer products, such as drink bottles, DVDs, eyeglass lenses, electronics, car parts, lining food cans and water pipes. BPA is on the ILFI [[Red list of Materials|Red list]] of building materials. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Revision as of 13:03, 3 November 2023
Description
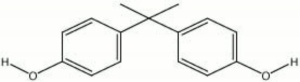
White flaky material with a phenolic odor. Bisphenol A (BPA) is one of the primary components in many Epoxy resins. It is also used to make Polycarbonates, Phenoxy resins, Polysulfone resins as well as some Flame retardants and Fungicides. The plastic resins containing BPA are used in consumer products, such as drink bottles, DVDs, eyeglass lenses, electronics, car parts, lining food cans and water pipes. BPA is on the ILFI Red list of building materials.
Synonyms and Related Terms
2,2-bis(4-hydroxyphenyl)propane
Risks
- Combustible
- ThermoFisher: SDS
Physical and Chemical Properties
Insoluble in water. Soluble in bases, ethanol, acetone.
| Composition | (CH3)2C(C6H4OH)2 |
|---|---|
| CAS | 80-05-7 |
| Melting Point | 150-155 C |
| Molecular Weight | mol. wt.=228.3 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1338
- Website: www.haz-map.com/allergic.htm