Difference between revisions of "Phenolphthalein"
(username removed) |
(username removed) |
||
| Line 41: | Line 41: | ||
== Authority == | == Authority == | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 184 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * S.R.Trotman, E.R. Trotman, ''Textile Analysis'', J.B. Lippincott Company, Philadelphia, 1932 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 | ||
| − | * | + | * Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 |
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: Solution: Dissolve 1 g phenolphthalein in 50 ml alcohol and add 50 ml water. | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: Solution: Dissolve 1 g phenolphthalein in 50 ml alcohol and add 50 ml water. | ||
Revision as of 07:32, 24 July 2013
Description
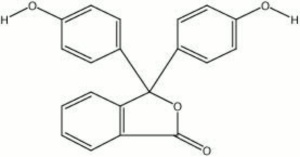
Pale yellow powder that is used as a pH indicator. Phenolphthalein is a triphenylmethane salt whose three aromatic rings produce a red chromophore in basic and neutral solutions. The conjugation is disrupted in acidic solutions resulting in a clear solution. Thus phenolphthalein is an effective acid base indicator for titrations of mineral acids, organic acids and alkalis. It is prepared as a 1% solution in ethanol. In solutions with a pH below 8.5, phenolphthalein is colorless and solutions above pH 9 are red.
Synonyms and Related Terms
3,3-bis(p-hydroxyphenyl)phthalide; 3,3-bis(4-hydroxyphenyl)-1(3H)-isobenzofuranore
Other Properties
Soluble in ethanol, ether and alkalis. Insoluble in water.
Solution: Dissolve 1 g phenolphthalein in 50 ml ethanol and add 50 ml water.
| Composition | (C6H4OH)2C2O2C6H4 |
|---|---|
| CAS | 77-09-8 |
| Melting Point | 258-262 |
| Density | 1.299 |
| Molecular Weight | mol. wt. = 318.33 |
Hazards and Safety
Toxic by ingestion, even small amounts will cause illness. Suspected carcinogen. Contact may cause irritation.
Mallinckrodt Baker: MSDS
Authority
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 184
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- S.R.Trotman, E.R. Trotman, Textile Analysis, J.B. Lippincott Company, Philadelphia, 1932
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: Solution: Dissolve 1 g phenolphthalein in 50 ml alcohol and add 50 ml water.