Difference between revisions of "Anatase"
(username removed) |
|||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | One of three naturally occuring isomorphic forms of [ | + | One of three naturally occuring isomorphic forms of [[titanium%20dioxide|titanium dioxide]]: anatase, rutile, and brookite. Anatase forms hard, transparent crystals. Deposits have been found in the Alps, Brazil, and the Ural Mountains; it is also formed by the weathering of titanite. Anatase was produced synthetically for use as a white paint pigment in 1906. It was a common white pigment in the early 20th century. Once the first commercially viable method for producing rutile was developed in 1938, production shifted to rutile because white paints with anatase pigments were subject to chalking and yellowing. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Revision as of 12:34, 7 January 2014
Description
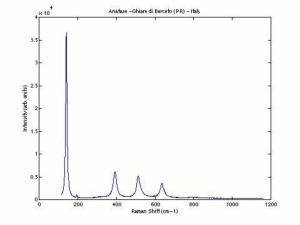
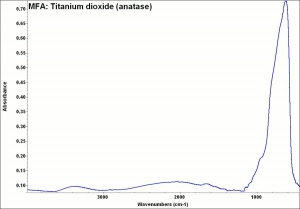
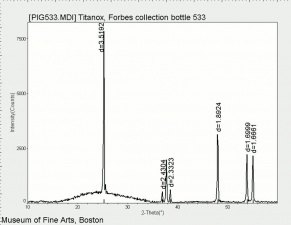
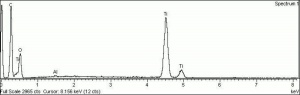
One of three naturally occuring isomorphic forms of Titanium dioxide: anatase, rutile, and brookite. Anatase forms hard, transparent crystals. Deposits have been found in the Alps, Brazil, and the Ural Mountains; it is also formed by the weathering of titanite. Anatase was produced synthetically for use as a white paint pigment in 1906. It was a common white pigment in the early 20th century. Once the first commercially viable method for producing rutile was developed in 1938, production shifted to rutile because white paints with anatase pigments were subject to chalking and yellowing.
Synonyms and Related Terms
octahedrite; titanium dioxide; anatase (Eng., Fr., Port., Nor.); anastase (sp); Anatas (Deut.); anatasio (It.); anatasa (Esp.); anataas (Ned.);
Other Properties
Tetragonal crystal system. Particle size 0.2 - 0.5 micrometers.
At high temperatures anatase will convert to rutile
High birefringence under crossed polars. Weak white fluorescence.
| Composition | TiO2 |
|---|---|
| Mohs Hardness | 5.0 - 6.0 |
| Melting Point | 1560 |
| Density | 3.88-3.94 |
| Refractive Index | 2.54 - 2.55 |
Hazards and Safety
Nontoxic. No significant hazards.
Acts as photocatalysts when exposed to UV light
Additional Information
° M.Laver, "Titanium Dioxide Whites", Artists Pigments, Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997. ° Walter C. McCrone, "Polarized Light Microscopy in Conservation: A Personal Perspective" JAIC 33(2):101-14, 1994. (contains a table of dates on the history of titanium white as a pigment)
Comparisons
Characteristics of Common White Pigments
Authority
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 815
- Encyclopedia Britannica, http://www.britannica.com Comment: "anatase" Encyclopædia Britannica [Accessed January 22, 2002].;
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: Ref. Index = 2.5; density = 3.9
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980 Comment: Ref. index = 2.56, 2.49
- Artists' Pigments: A Handbook of their History and Characteristics, Elisabeth West FitzHugh, Oxford University Press, Oxford, Vol. 3, 1997 Comment: M.Laver, "Titanium Dioxide Whites"
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Anatase
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Website address 1 Comment: Pigments Through the Ages at http://webexhibits.org/pigments : Refractive index: anatase: 2.3 - 2.65