Difference between revisions of "Malachite Green"
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
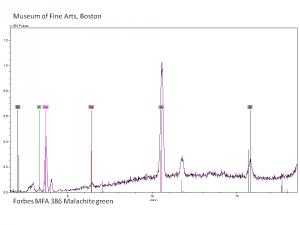
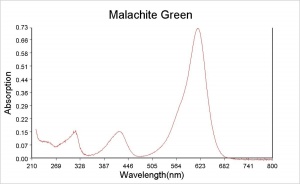
| − | A synthetic organic dye that was named for the mineral because of its color hue. Malachite Green is an aniline based dyestuff (a triphenylmethane salt) made by Dobner and Fisher in 1877. Both the chloride and the oxalate salt are known by the same name. In a neutral aqueous solution, its green crystals give an absorption maximum at 616.9 nm. Malachite Green is used for the direct dying of [ | + | A synthetic organic dye that was named for the mineral because of its color hue. Malachite Green is an aniline based dyestuff (a triphenylmethane salt) made by Dobner and Fisher in 1877. Both the chloride and the oxalate salt are known by the same name. In a neutral aqueous solution, its green crystals give an absorption maximum at 616.9 nm. Malachite Green is used for the direct dying of [[silk|silk]], [[wool|wool]], [[jute|jute]], and [[leather|leather]]. It is also used to dye [[cotton|cotton]] after mordanting. Malachite Green can be used in spot chemical tests for the detection of sulfurous acid and [http://cameo.mfa.org/materials/fullrecord.asp?name=cerium cerium]. It is used as an acid-base indicator with the following color ranges: yellow at pH 0, green at pH 2.0, cyan at pH 11.6, and colorless at pH 14. Malachite Green is also used to kill parasites, mold, and [[fungus|fungi]] in fish tanks. |
Revision as of 08:56, 9 May 2014
Description
A synthetic organic dye that was named for the mineral because of its color hue. Malachite Green is an aniline based dyestuff (a triphenylmethane salt) made by Dobner and Fisher in 1877. Both the chloride and the oxalate salt are known by the same name. In a neutral aqueous solution, its green crystals give an absorption maximum at 616.9 nm. Malachite Green is used for the direct dying of Silk, Wool, Jute, and Leather. It is also used to dye Cotton after mordanting. Malachite Green can be used in spot chemical tests for the detection of sulfurous acid and cerium. It is used as an acid-base indicator with the following color ranges: yellow at pH 0, green at pH 2.0, cyan at pH 11.6, and colorless at pH 14. Malachite Green is also used to kill parasites, mold, and fungi in fish tanks.
Synonyms and Related Terms
malachite green chloride; Basic Green 4; CI 42000; viktoriavihreä (Fin.); Viktoriagrün (Deut.); Braunschweigergrün (Deut.); vert malachite (Fr.); viktoriagrönt (Sven.); verde malaquite (Port.); benzaldehyde green;
COmmercial names: Victoria Green B; China Green; Diamond Green B; Light Green N; Benzal Green; Fast Green
Other Properties
Soluble in water and ethanol, methanol and amyl alcohol.
| Composition | C23H25ClN2 |
|---|---|
| CAS | 569-64-2 |
| Molecular Weight | mol. wt. = 364.92 |
Hazards and Safety
Toxic by ingestion. Contact may cause irritation.
Fisher Scientific: MSDS
Authority
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5739
- Encyclopedia Britannica, http://www.britannica.com Comment: "malachite green." Encyclopædia Britannica. 2005. Encyclopædia Britannica Premium Service 7 Apr. 2005 .
- Website address 1 Comment: www.straw.com/sig/dyehist
- Website address 2 Comment: http://www.coloria.net/varita.htm - foreign language equivalent terms
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996
- Colour Index International online at www.colour-index.org