Difference between revisions of "Aleuritic acid"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 35: | Line 35: | ||
Fisher Scientific: [http://www.fishersci.ca/msds2.nsf/0/D5714E8F71C60AA685256CA40083BE10/$file/MSDS-56729.html?open MSDS] | Fisher Scientific: [http://www.fishersci.ca/msds2.nsf/0/D5714E8F71C60AA685256CA40083BE10/$file/MSDS-56729.html?open MSDS] | ||
| − | == | + | == Sources Checked for Data in Record == |
* ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 222; mp = 100-101 C | * ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 222; mp = 100-101 C | ||
Revision as of 12:13, 29 April 2016
Description
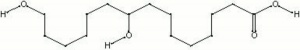
A constituent of Shellac composing approximately 40%. Aleuritic acid forms a complex polyester upon esterification. It is also used in perfumes and chemical synthesis. Synthetically prepared aleuritic acid is often used as a component in Cellulose nitrate lacquers. It can also act as a surfactant.
Synonyms and Related Terms
9,10,16-trihydroxyhexadecanoic acid; 9,10,16-trihydroxypalmitic acid
Other Properties
Soluble in ethanol and methanol.
| Composition | C16H32O5 |
|---|---|
| CAS | 533-87-9 |
| Melting Point | 100-101 |
| Density | 1.114 |
| Molecular Weight | mol. wt. = 304.41 |
Hazards and Safety
Fisher Scientific: MSDS
Sources Checked for Data in Record
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 222; mp = 100-101 C
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 706
- C.V.Horie, Materials for Conservation, Butterworth-Heineman, London, 1997
- MSDS Sheet Comment: Fisher Scientific - mp= 100-105 C