Difference between revisions of "Ammonium phosphate dibasic"
Jump to navigation
Jump to search
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
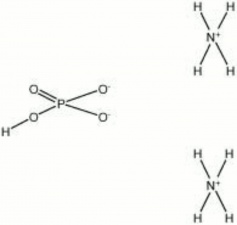
| − | + | [[[SliderGallery rightalign|ammonium phosphate dibasic.jpg~Chemical structure]]] | |
| − | + | Ororless, colorless crystals that is used to fireproof textiles, paper, wood, and baskets. Ammonium phosphate dibasic is also used as a flux for soldering [[tin|tin]], [[copper|copper]], [[brass|brass]], and [[zinc|zinc]]. | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 7: | Line 7: | ||
secondary ammonium phosphate; diammonium hydrogen phosphate | secondary ammonium phosphate; diammonium hydrogen phosphate | ||
| − | + | == Risks == | |
| − | == | + | * Harmful if swallowed. |
| − | + | * Contact causes irritation. | |
| − | + | * Fisher Scientific: [https://www.fishersci.com/store/msds?partNumber=AC447140010&productDescription=AMMONIUM+PHOSPHATE%2C+DIBA+1KG&vendorId=VN00032119&countryCode=US&language=en SDS] | |
| − | + | == Physical and Chemical Properties == | |
| − | Used for the detection of magnesium ( | + | * Soluble in water forming solution with pH about 8. |
| + | * Insoluble in alcohol, acetone. | ||
| + | * Used for the detection of magnesium (Arnold 1984). | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 26: | Line 28: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.62 | + | | 1.62 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 32: | Line 34: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * A. Arnold, "Determination of mineral salts from monuments", Studies in Conservation (29) 1984 (Detection of magnesium) | |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 48: | Line 42: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 576 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 576 | ||
| − | + | * Wikipedia: http://en.wikipedia.org/wiki/Ammonium_phosphate (Accessed Mar. 20, 2006) | |
| − | |||
| − | * Wikipedia | ||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 14:34, 26 April 2022
Description
Ororless, colorless crystals that is used to fireproof textiles, paper, wood, and baskets. Ammonium phosphate dibasic is also used as a flux for soldering Tin, Copper, Brass, and Zinc.
Synonyms and Related Terms
secondary ammonium phosphate; diammonium hydrogen phosphate
Risks
- Harmful if swallowed.
- Contact causes irritation.
- Fisher Scientific: SDS
Physical and Chemical Properties
- Soluble in water forming solution with pH about 8.
- Insoluble in alcohol, acetone.
- Used for the detection of magnesium (Arnold 1984).
| Composition | (NH4)2HPO4 |
|---|---|
| CAS | 7783-28-0 |
| Density | 1.62 g/ml |
| Molecular Weight | mol. wt. = 132.06 |
Resources and Citations
- A. Arnold, "Determination of mineral salts from monuments", Studies in Conservation (29) 1984 (Detection of magnesium)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 576
- Wikipedia: http://en.wikipedia.org/wiki/Ammonium_phosphate (Accessed Mar. 20, 2006)