Difference between revisions of "Barium yellow"
| Line 65: | Line 65: | ||
* Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 | * Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 | ||
| − | * Art and Architecture Thesaurus Online, | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 08:53, 2 May 2022
Description
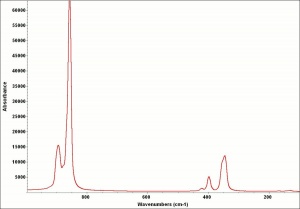
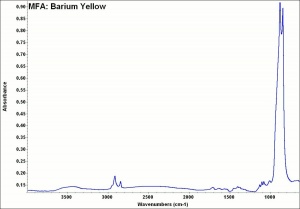
A banana-color yellow pigment composed of Barium chromate. Barium chromate is precipitated from a Barium chloride solution by the addition of Potassium dichromate. Barium yellow is a fairly permanent pigment but it may turn green slowly in light. It has low tinting strength and opacity. It was not widely used as an artists pigment by itself but was sometimes mixed with Strontium yellow and Zinc yellow then sold under the name lemon yellow or citron yellow. It was also used for coloring glass, and ceramic glazes. Currently barium chromate is used in anticorrosion pastes and in metal primers.
Synonyms and Related Terms
Pigment Yellow 31; CI 77103; Barytgelb (Deut.); amarillo de bario (Esp.); cromato de bario (Esp.); jaune d'outremer (Fr.); jaune de baryum (Fr.); giallo di bario (It.); amarelo de bário (Port.); ultramarine yellow; lemon yellow; permanent yellow; barium chromate yellow; baryta yellow; Steinbuhl yellow
Risks
Toxic by inhalation and ingestion. Skin contact may cause irritation.
Carcinogenic. Combustible. Decomposed by heat.
CDH Fine Chemicals: SDS
Physical and Chemical Properties
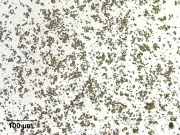
Soluble in dilute mineral acids and alkalis. Insoluble in water. Microscopically, it may appear as colorless, birefracting, rhombic plates,
| Composition | BaCrO4 |
|---|---|
| CAS | 10294-40-3 |
| Density | 4.45-4.49 g/ml |
| Molecular Weight | mol. wt. = 253.32 |
| Refractive Index | 1.94 - 1.98 |
Resources and Citations
- H. Kuhn, M.Curran, "Chrome Yellow and Other Chromate Pigments", Artists Pigments, Volume 1, R. Feller (ed.), Cambridge University Press: Cambridge, 1986.
- Pigments Through the Ages - http://webexhibits.org/pigments
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- M. Doerner, The Materials of the Artist, Harcourt, Brace & Co., 1934
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry #999
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000