Difference between revisions of "Soda ash"
Jump to navigation
Jump to search
| Line 10: | Line 10: | ||
== Risks == | == Risks == | ||
| − | Noncombustible. Corrosive to skin and eyes. | + | * Noncombustible. |
| − | + | * Corrosive to skin and eyes. | |
| − | JMN Specialties: [https://www.jmnspecialties.com/downloads/sds/2099-soda-ash-sds/file SDS] | + | * JMN Specialties: [https://www.jmnspecialties.com/downloads/sds/2099-soda-ash-sds/file SDS] |
== Physical and Chemical Properties == | == Physical and Chemical Properties == | ||
| − | Soluble in water, glycerol. Insoluble in ethanol. Aqueous solution has a pH = 11.6. | + | * Soluble in water, glycerol. Insoluble in ethanol. |
| + | * Aqueous solution has a pH = 11.6. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 26: | Line 27: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 851 | + | | 851 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.53 | + | | 2.53 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
Latest revision as of 13:47, 31 May 2022
Description
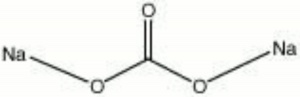
A white to gray powder of Sodium carbonate. Soda ash naturally occurs as trona in mineral water deposits. Impurities may include Sodium chloride, sodium sulfate, Sodium bicarbonate, Calcium carbonate, and Magnesium carbonate. Soda ash is made synthetically by the Solvay process. Soda ash is used primarily in the manufacture of Glass and Paper pulp. It is also used in soaps, water softeners, and Textile processing.
Synonyms and Related Terms
anhydrous sodium carbonate; calcined soda; Solvay soda; trona
Risks
- Noncombustible.
- Corrosive to skin and eyes.
- JMN Specialties: SDS
Physical and Chemical Properties
- Soluble in water, glycerol. Insoluble in ethanol.
- Aqueous solution has a pH = 11.6.
| Composition | Na2CO3 |
|---|---|
| CAS | 497-19-8 |
| Melting Point | 851 C |
| Density | 2.53 g/ml |
| Molecular Weight | mol. wt. = 105.99 |
Resources and Citations
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 734
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 8739
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997