Lithium carbonate: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 2: | Line 2: | ||
A white crystalline compound. Lithium carbonate is used as a [[flux]] in the manufacture of [[ceramic]] and [[porcelain]] [[glaze|glazes]], and [[enamel, inorganic|enamels]]. It is also used to make [[luminescence|luminescent]] [[paint|paints]], [[varnish|varnishes]], and [[dye|dyes]]. | A white crystalline compound. Lithium carbonate is used as a [[flux]] in the manufacture of [[ceramic]] and [[porcelain]] [[glaze|glazes]], and [[enamel, inorganic|enamels]]. It is also used to make [[luminescence|luminescent]] [[paint|paints]], [[varnish|varnishes]], and [[dye|dyes]]. | ||
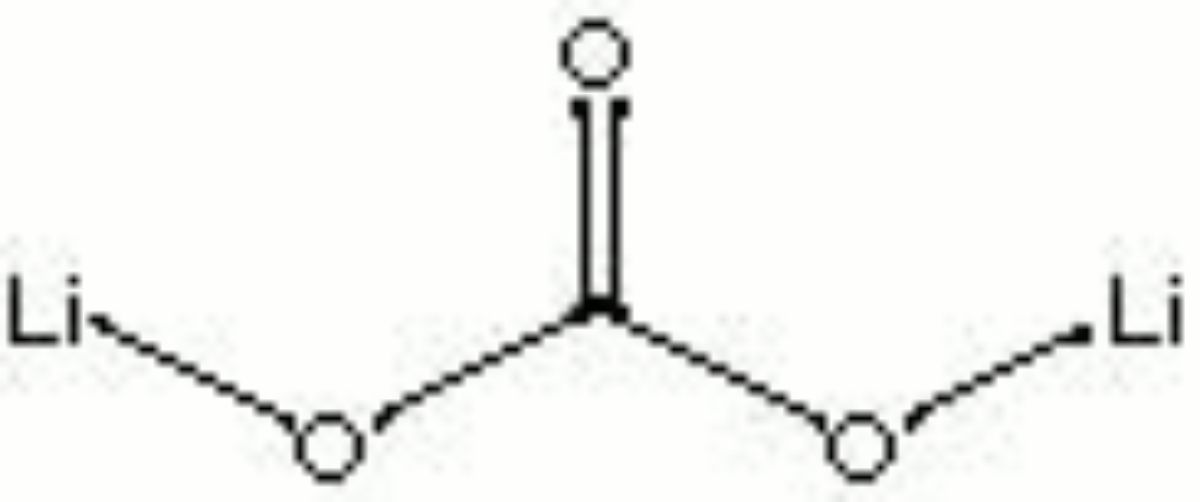
[[[SliderGallery rightalign|lithium carbonate.jpg~Chemical structure]]] | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
dilithium carbonate; Camcolit; Candamide; Carbolith; Eskalith; Limas; Lithane; Lithobid; Lithonate; Lithotabs; Plenur; Priadel | dilithium carbonate; Camcolit; Candamide; Carbolith; Eskalith; Limas; Lithane; Lithobid; Lithonate; Lithotabs; Plenur; Priadel | ||
== Risks == | == Risks == | ||
Latest revision as of 11:07, 16 September 2022
Description
A white crystalline compound. Lithium carbonate is used as a flux in the manufacture of ceramic and porcelain glazes, and enamels. It is also used to make luminescent paints, varnishes, and dyes.
Synonyms and Related Terms
dilithium carbonate; Camcolit; Candamide; Carbolith; Eskalith; Limas; Lithane; Lithobid; Lithonate; Lithotabs; Plenur; Priadel
Risks
- Corrosive to skin, eyes, and membranes.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in dilute acid. Slightly soluble in water. Insoluble in ethanol.
| Composition | Li2CO3 |
|---|---|
| CAS | 554-13-2 |
| Melting Point | 720 C |
| Density | 2.111 g/ml |
| Molecular Weight | mol. wt. = 73.9 |
| Boiling Point | 1200 C (dec) |
Resources and Citations
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 5552
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979