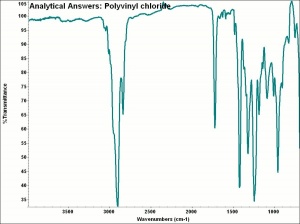
Polyvinyl chloride
Description
A colorless thermoplastic polymer made from vinyl chloride. Polyvinyl chloride (PVC) was discovered in 1838 Henri Regnault noticed a powder inside of a container of vinyl chloride. It was further studied in 1872 by Baumann and in 1912 by Ostromislensky, but was not commercialized until 1926, when a chemist at B.F.Goodrich, Waldo Semon, discovered a method to plasticize PVC into a soft rubbery material. The first moldable PVC, called Koroseal, was introduced in 1930. PVC is resistant to moisture but stabilizers are needed to prevent discoloration from light and heat. Lightly plasticized PVC has been used for gramophone records. Heavily plasticized PVC is used as a rubber substitute for thin sheeting, wire coverings, gaskets, tubing, raincoats, storage sleeves and fabric coatings. Phthalate plasticizers were commonly used in the middle of the 20th century for PVC plastics. However, these oily plasticizers tended to creep and separate with time producing an oily surface and leaving a brittle substrate so some more recent PVC formulations use copolymerization techniques for film modification rather than plasticizers. Rigid unplasticized PVC was introduced in 1958 in Europe as alternative piping for town water services to replace corroded iron pipes.
Synonyms and Related Terms
PVC; vinyl; polyvinylchloride; poli(cloruro de vinilo) (Esp.); chlorure de polyvinyle (Fr.); polivinil cloruro (It.); policloreto de vinilo (Port.); poly(vinyl chloride); poly vinylchloride (sp); chloroethene polymer; vinyl chloride plastic; poly(chlorethylene)
Examples: Geon [B.F.Goodrich]; Koroseal [B.F.Goodrich]; Tygon; Vinagel; Elaston; Trovidur; Bexan [BX Plastics]; Bristrand [Polymers Inc.]; Pe-Ce-U [Bayer].; Tricovil; Kubo
Other Properties
Soluble in chlorinated hydrocarbons and aromatic solvents.
Insoluble in water, alcohols, concentrated acids and alkalis.
Burns with green, smoky flame and evolves HCl; it extinguishes when removed from flame source.
| Composition | [-H2CCHCl-]n |
|---|---|
| CAS | 9002-86-2 |
| Melting Point | 148 (dec) |
| Density | 1.406 |
| Refractive Index | 1.54 |
Hazards and Safety
Degrades with heat and light to darken and potentially produce hydrochloric acid. May also form carbon monoxide or phosgene. Plasticizers and stabilizers can continually ooze from polymer resulting in an oily film, white bloom, or corroded metal. Additionally some plasticizers are toxic. PVC is a suspected carcinogen.
Production and incineration of PVC can introduce toxic chlorinated organic chemicals into the environment (e.g. dioxin).
Fisher Scientific: MSDS
Comparisons
General Characteristics of Polymers
Physical Properties for Selected Thermoplastic Resins
Additional Images
Authority
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- J.Gordon Cook, Handbook of Textile Fibres:II Man-made Fibres, Merrow Publishing Co. , Durham, England Comment: p. 444; discovered by Henri Regnault in 1838
- M.Kaufman, The First Century of Plastics, The Plastics and Rubber Institute, London, 1963
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- Marjorie Shelley, The Care and Handling of Art Objects, The Metropolitan Museum, New York, 1987
- Caring for your Collections, Arthur W Schulz (ed.), Harry N. Abrams, Inc. , New York, 1992
- Sharon Blank, An introduction to plastics and rubbers in collections, Studies in Conservation, 35, 53-63, 1990
- M. Baker, E. McManus, 'History, Care and Handling of America's Spacesuits', JAIC, 31, 77-85, 1992
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Website address 1 Comment: www.nswpmith.com.au/historyofplastics.html - discovered by Henri Regnault in 1838.