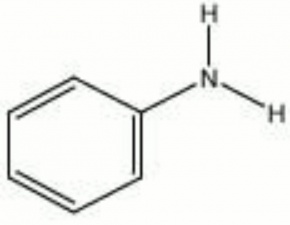
Aniline
Jump to navigation
Jump to search
Description
A colorless, oily liquid with a sweet amine-like odor. Aniline was first isolated in 1826 by Unverdorben and made synthetically in 1841 by Fritzsche. It was the base material for many of the early synthetic dyes. As a result, colors produces from coal-tar derivatives are generally called aniline dyes. Aniline darkens to brown on exposure to air and light. Besides the manufacture of dyes, aniline is used as an Accelerator and Antioxidant in rubbers. It is also used to make Polyurethane foams, fungicides, and explosives.
Synonyms and Related Terms
aminobenzene; benzenamine; phenylamine; aminophen; aniline oil; krstallin; kyanol
Risks
- Highly toxic by ingestion, inhalation and skin absorption.
- Combustible forming toxic nitrogen oxides and aniline vapor.
- Fisher Scientific: SDS
Physical and Chemical Properties
Soluble in ethanol, ether and benzene. Slightly soluble in water.
| Composition | C6H5NH2 |
|---|---|
| CAS | 62-53-3 |
| Melting Point | -6.2 C |
| Density | 1.022 g/ml |
| Molecular Weight | mol. wt. = 93.1 |
| Refractive Index | 1.583 |
| Boiling Point | 184-186 C |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 59
- Encyclopedia Britannica, http://www.britannica.com Comment: "aniline" [Accessed May 22, 2003].
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988 Comment: [http:/www.ekornes.com/stressless/leaglos1.html/ Link]
- Laboratory Safety Information: Link
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.583