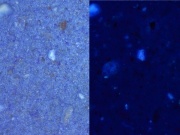
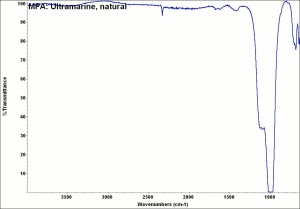
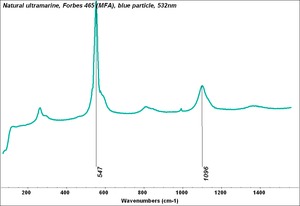
Ultramarine blue, natural
Description
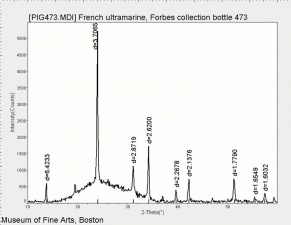
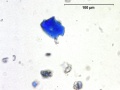
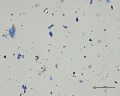
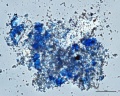
Natural ultramarine blue pigment is the ground, separated blue particles (Lazurite) from the gemstone Lapis lazuli. Lapis has been mined since the 3rd millennium BCE in Afghanistan at the Badakhshan mines. Ancient trade routes distributed the lapis throughout Europe and the Far East. From the 13th century, the method used to prepare ultramarine pigment mixed powdered lapis with wax, resin and oils then kneaded the mixture in a dilute lye solution; this allowed the blue particles to disperse into the alkaline water while the extraneous minerals (e.g., Calcite, Pyrite, silicates) are retained in the putty. This was a time consuming process that produced varying shades of blue. The purest, deep blue was extracted in the first batch and sold for very high prices. As the process was continued, the subsequent batches of pigments were slightly less pure; the final batch was a transparent blue gray called ultramarine ash. In all the fractions, some transparent material is present (calcite) which aids microscopically in the distinguishing the natural from synthetic ultramarine pigments which were first made in 1828. Ultramarine blue is used as a pigment in paints ( oil, Tempera, watercolor), Wallpaper, Soap, textile printing, and laundry bluing agents.
Synonyms and Related Terms
natural ultramarine blue; Pigment blue 29; CI 77007; lapis lazuli blue; Ultramarinblau, echt (Deut.); outremer naturel (Fr.); blu oltremare naturale (It.); lapislazzuli (It.); ultramarino verdadero (Esp.); lapislázuli (Esp.); oyltramarina fysikh (Gr.); lapis lazuli (Gr.); ultramarijn blauw (Ned.); ultramaryna (Pol); ultramarin (Sven.); azul ultramarino, natural (Port.); bleu d'Azur; Armenian blue; lazuline blue; ultramarine ash; mineral blue; vein stone; lazurstein
Risks
- No significant hazards.
- Noncombustible.
- Becomes dull with outdoor weathering.
Physical and Chemical Properties
- Discolors when exposed to weak acids or sulfur fumes.
- Irregular particles, no birefringence, no pleochroism, extinction under crossed polars.
- Naturally mixed with silicates, calcite and pyrite.
- Insoluble in water.
- Isotropic crystal system.
- ASTM lightfastness=1 (excellent)
| Composition | 3Na2O.3Al2O3.6SiO2.2Na2S |
|---|---|
| Density | 2.4 - 2.5 g/ml |
| Refractive Index | about 1.5 |
Comparisons
Characteristics of Common Blue Pigments
Additional Images
Resources and Citations
- J. Plesters, "Ultramarine Blue, Natural and Artificial", Artists Pigments, Volume 2, A. Roy (ed.), Oxford University Press: Oxford, 1993.
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- Encyclopedia Britannica, http://www.britannica.com Comment: "ultramarine." Accessed 7 Apr. 2005 .
- Pigments through the ages -http://webexhibits.org/pigments/indiv/technical/ultramarine.html
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments"
- Colour Index International online at www.colour-index.org
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000