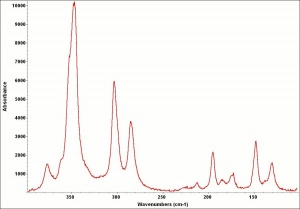
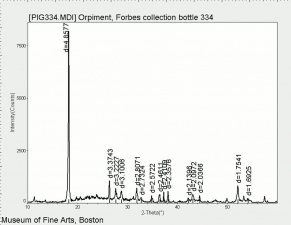
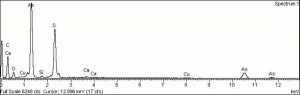
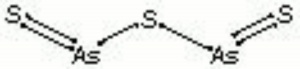Orpiment
Description
A soft, yellow mineral composed of arsenic trisulfide. Orpiment occurs naturally in volcanic fumaroles, hydrothermal veins, hot springs, and as a decomposition product of realgar. Deposits are found in the Czech Republic, Romania (Copalnic), Germany (Andreas-Berg ), Switzerland (Valais), Turkey (Çölemerik), Macedonia, Japan and the United States (Utah, Nevada, Wyoming). Orpiment ranges in color from a bright lemon yellow to orange. It changes to the red crystalline form at 170C. Orpiment was used in many early civilizations at various times, such as Egypt, Syria, Persia, India, and China. It was used as a pigment in European painting from quite early times, including in manuscript illumination and polychrome sculpture, and became almost a standard material on the palette in Venice in the 16th-century. Elsewhere it occurs in Dutch 17th-century painting, particularly flowerpieces, and British and French 18th-century paintings. Its use continued almost up until the present day, though it is no longer commonly used due to its toxicity. Orpiment has good tinting strength but is not considered permanent as it reacts with copper pigments as well as some lead pigments to produce dark copper or lead sulfides. It is a poor drier for oil paints and has a sulfurous odor. It can be rather light sensitive, losing its color on prolonged exposure to light, particularly in aqueous media. Arsenic trisulfide was made synthetically in the 18th-century and sold as king's yellow. The synthetic variety was purer and less expensive.
Synonyms and Related Terms
arsenic trisulfide; Pigment Yellow 39; CI 77085, 77086; orpiment (Eng., Fr., Gr., Ned.); Auripigment (Deut.); Rauschgelb (Deut.); Konigsgelb (Deut.); jaune royal (Fr.); oropimente (Esp.); orpimento (It.); ouropigmento (Port.); arsenous sulfide; king's yellow; arsenic yellow; auripigmentum; Chinese yellow; sunflower yellow
Other Properties
Decomposes slowly in water; soluble in acids and alkalis.
Unstable when mixed with alkaline pigments such as in buon fresco or in combinations with lime white.
Both orpiment and realgar lose color on exposure to light. Orpiment oxidizes to form translucent or white oxides of arsenic.
Monoclinic with poorly formed crystals. The mineral variety often shows fairly large crystalline tabular particle with striations and a waxy appearant. Cleavage is good in one direction.
Luster = pearly to resinous. Streak = lemon-yellow.
Pigment particles are strongly anisotropic. The small, often elongated, yellow particles (4-20 microns) exhibit high birefringence and straight extinction.
| Composition | As2S3 |
|---|---|
| CAS | 1303-33-9 |
| Mohs Hardness | 1.5 - 2.0 |
| Melting Point | 300 |
| Density | 3.43 |
| Molecular Weight | mol. wt. = 246.04 |
| Refractive Index | 2.40; 3.02; 2.81 |
Hazards and Safety
Turns black in contact with copper and lead containing pigments.
Toxic by inhalation and ingestion.
Fisher Scientific: MSDS
Additional Information
° E.West FitzHugh, "Orpiment and Realgar", Artists Pigments, Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997. ° Mineralogy Database: Orpiment° Carolin Rötter, ‘Auripigment’, Restauro, 6 2003, 408-413.Record content reviewed by EU-Artech, November 2007.
Additional Images
Authority
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- External source or communication Comment: Submitted information: Ashok Roy, November 2007
Submitted information: Helen Howard, November 2007
- External source or communication Comment: Submitted information: Ashok Roy, Helen Howard, November 2007
- Artists' Pigments: A Handbook of their History and Characteristics, Elisabeth West FitzHugh, Oxford University Press, Oxford, Vol. 3, 1997 Comment: E.West FitzHugh, "Orpiment and Realgar"
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- R.Feller, M.Curran, C.Bailie, 'Identification of Traditional Organic Colorants Employed in Japanese Prints and Determination of their Rates of Fading', Japanese Woodblock Prints, Allen Memorial Art Museum, Oberlin College, Oberlin, 1984 Comment: Jap. name kio and sekio and stone yellow
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 69
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Encyclopedia Britannica, http://www.britannica.com Comment: "orpiment." Encyclopædia Britannica. 2005. Encyclopædia Britannica Premium Service 7 Apr. 2005 .
- Website address 1 Comment: Pigments Through the Ages - http://webexhibits.org/pigments/indiv/technical/orpiment.html
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Arsenic_trisulfide (accessed Aug 30 2005)
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000