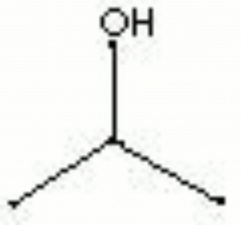
Isopropyl alcohol
Revision as of 06:31, 24 July 2013 by (username removed)
Description
Colorless liquid with a pleasant smell. Isopropyl alcohol, or isopropanol, is used as a solvent for gums, shellac, nondrying oils, natural resins, and inks. It is also used as an antiseptic in cleansers and body lotions. Isopropanol is added to ethanol as a denaturant.
Synonyms and Related Terms
isopropanol; rubbing alcohol; IPA; dimethylcarbinol; sec-propyl alcohol; 2-propanol
Other Properties
Miscible with water, ethanol, ether, chloroform. Insoluble in salt solutions.
| Composition | (CH3)2CHOH |
|---|---|
| CAS | 67-63-0 |
| Melting Point | -86 |
| Density | 0.7863 |
| Molecular Weight | mol. wt.=60.1 |
| Refractive Index | 1.3756 |
| Boiling Point | 82.4 |
Hazards and Safety
Flammable. Dangerous fire risk. Flash point = 12 C (54 F)
Skin contact may cause dryness and irritation. Toxic by ingestion and inhalation.
Mallinckrodt Baker: MSDS
Comparisons
Authority
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5227; ref. index=1.3756
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.375