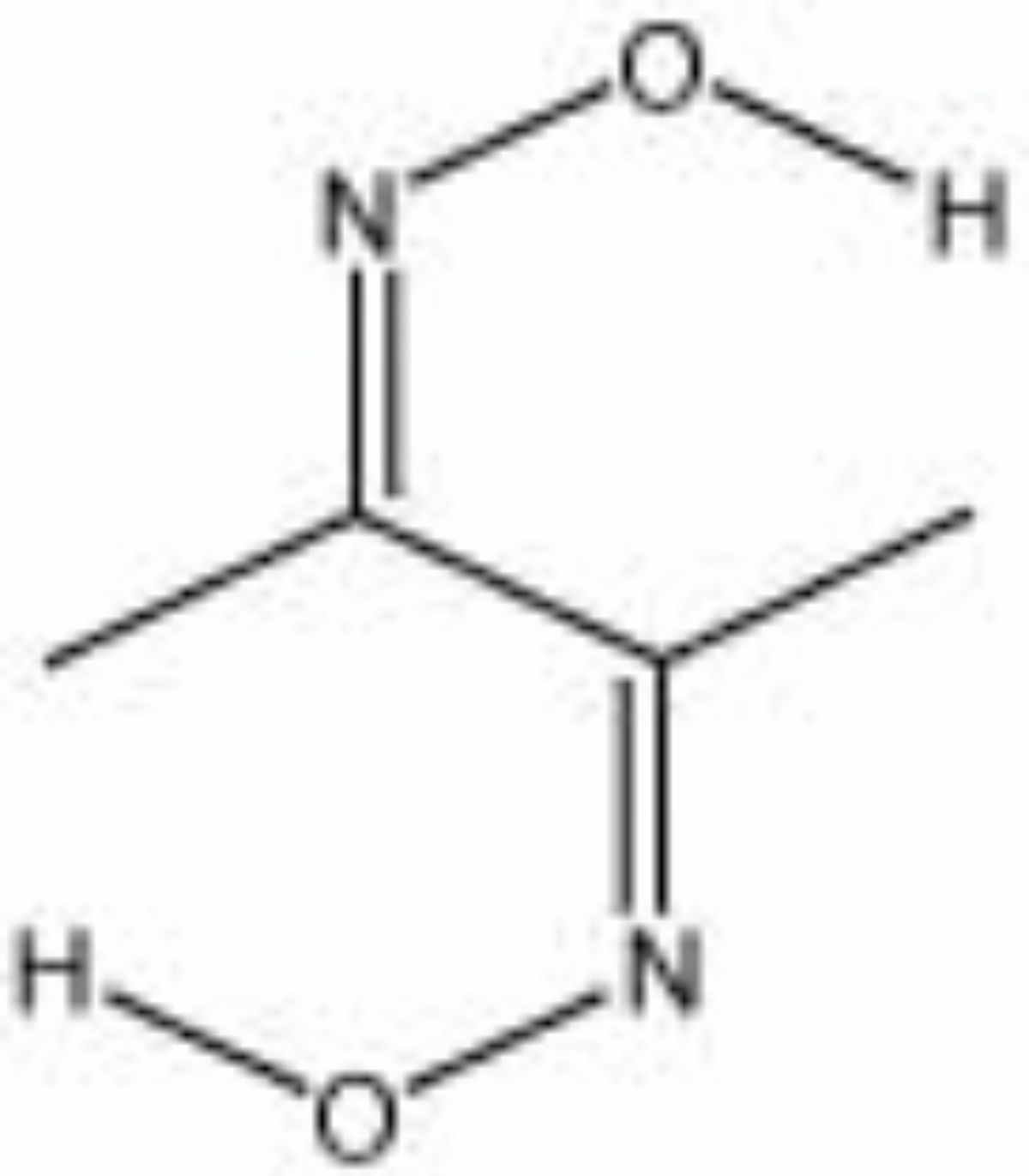
Dimethylglyoxime
Description
Transparent triclinic crystals used in a colorimetric reagent to detect trace amounts of nickel, copper, cobalt, or bismuth (Odegaard et al 2000). Dimethylglyoxime reacts with soluble nickel to produce a bright red precipitate. Copper produces a blue precipitate, cobalt gives a brown, and bismuth forms a bright yellow.
Synonyms and Related Terms
2,3-butanedionedioxime; 2,3-diisonitrosobutane; diacetyldioxime
Other Properties
Insoluble in water. Soluble in alcohol, ether, pyridine, acetone.
Solution (0.01N): Dissolve 0.6 g dimethylglyoxime in 500 ml of 95% ethyl alcohol.
| Composition | C4H8N2O2 |
|---|---|
| CAS | 95-45-4 |
| Melting Point | 238-240 |
| Molecular Weight | mol. wt. = 116.12 |
Hazards and Safety
Harmful by ingestion. Contact may cause irritation.
Mallinckrodt Baker: MSDS
Additional Information
N.Odegaard, S.Carroll, W.Zimmt, Material Characterization Tests for Objects of Art and Archaeology Archetype Publications, London, 2000, p.80.
Sources Checked for Data in Record
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 3240
- N.Odegaard, S.Carroll, W.Zimmt, Material Characterization Tests for Objects of Art and Archaeology, Archetype Publications, London, 2000
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: Solution (0.01N): Dissolve 0.6 g dimethylglyoxime in 500 ml of 95% ethyl alcohol.
- Sigma Dyes, Stains and Natural Pigments, Infrared Library, Nicolet, 1991-1995 Comment: OMNIC: formula= C4H8N2O2, CAS= 95-45-4