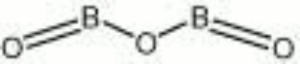Boric oxide
Revision as of 06:27, 24 July 2013 by (username removed)
Description
A colorless crystalline compound used in metallurgy and in the manufacture of heat-resistant (borosilicate) glassware. Boric oxide is obtained from boric acid, borax, sodium borate, or colemanite. It is used in combination with silica as a flux for glazes.
Synonyms and Related Terms
boron oxide; boric anhydride; boron trioxide; boron sesquioxide
Other Properties
Soluble in ethanol, hot water.
| Composition | B2O3 |
|---|---|
| CAS | 1303-86-2 |
| Melting Point | 450 |
| Density | 2.46 |
| Molecular Weight | mol. wt. = 69.6 |
| Boiling Point | 1500 |
Hazards and Safety
Noncombustible.
LINK: International Chemical Safety Card
Authority
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Henry Hodges, Artifacts: An Introduction to Early Materials and Technology, Ronald P. Frye, Kingston, Canada, 1988
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997