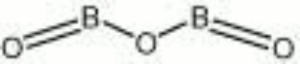Boric oxide
Jump to navigation
Jump to search
Description
A colorless crystalline compound used in metallurgy and in the manufacture of heat-resistant (borosilicate) glassware. Boric oxide is obtained from Boric acid, Borax, Sodium borate, or Colemanite. It is used in combination with silica as a flux for glazes.
Synonyms and Related Terms
boron oxide; boric anhydride; boron trioxide; boron sesquioxide
Risks
Noncombustible.
Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in ethanol, hot water.
| Composition | B2O3 |
|---|---|
| CAS | 1303-86-2 |
| Melting Point | 450 C |
| Density | 2.46 g/ml |
| Molecular Weight | mol. wt. = 69.6 |
| Boiling Point | 1500 C |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Henry Hodges, Artifacts: An Introduction to Early Materials and Technology, Ronald P. Frye, Kingston, Canada, 1988
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997