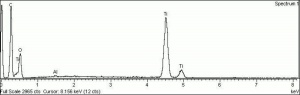
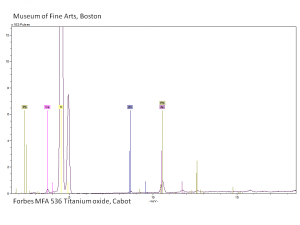
Titanium dioxide
Description
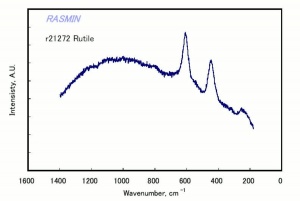
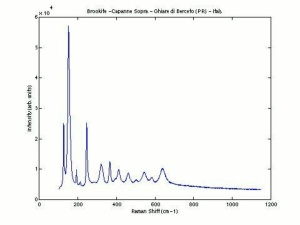
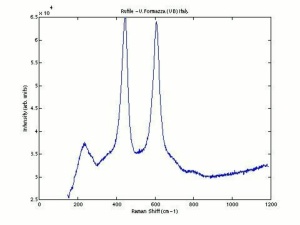
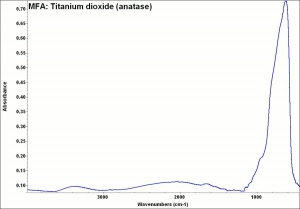
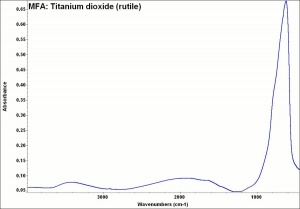
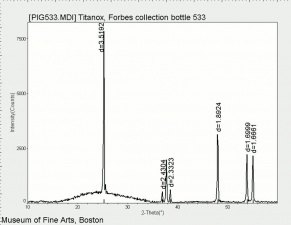
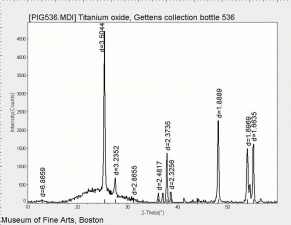
Titanium dioxide occurs naturally in three crystalline forms: Anatase, Rutile and Brookite, with rutile being the most abundant. In mineral form, titanium dioxide is often deeply coloured due to elemental impurities. Naturally occurring anatase and brookite are not used for pigment manufacture. Ground mineral rutile found some use as a colored pigment, but difficulties in grinding result in coarse particles with a different morphology than that of synthetic material. Both anatase and rutile have been synthesized since the early 20th century for use as white pigments, generally using processes based on either sulfuric acid or chlorine. The pigments are used in applications including paints, pastels, inks, paper, enamels, ceramics, glass, rubber, plastics, and as coatings and surface treatments in the textile and leather industries. They are nontoxic and can be used in foods and pharmaceuticals; their ultraviolet absorption properties have led to applications in sunscreen and lotions. Titanium dioxide is also used as a white reference material in many instrument dealing with optical measurement or color spectrometry. Rutile can be made into synthetic gemstones of varying color.
From about 1908 onward, researchers in Norway and the United States who were seeking to find a use for titanium-rich ore such as ilmenite (iron titanate) noted that titanium dioxide showed promise as a pigment due to its high hiding power. During development of a viable manufacturing method, small experimental batches of pigment were produced of varying composition and ranging in color from reddish yellow to off-white. Commercial manufacturing of anatase-based pigments began in Norway in 1918 (Titan Company A/S) and in the United States (Titanium Pigment Company), ramping up to full production from 1916 to 1919.
Synonyms and Related Terms
titanium white; rutile; anatase; brookite; Pigment White 6; CI 77891; dioxyde de titane (Fr.); Titandioxid (Deut., Sven.); bianco di titanio (It.); dióxido de titanio (Esp.); titandioksid (Nor.); dióxido de titânio (Port.); titania; titanic anhydride; titanic acid anhydride; titanic oxide; Unitane; Titanox
Other Properties
Particle size = 0.2-0.5 micrometers.
Chemically inert, insoluble in water, organic solvents, aqueous alkalis; can be dissolved in sulfuric or hydrofluoric acid; slow to dry in oil without additives or surface treatment.
Absorb strongly in the UV, are photochemically active and can cause deterioration of admixed media if exposed to ultraviolet light.
| Composition | TiO2 |
|---|---|
| CAS | 13463-67-7 |
| Mohs Hardness | 5.5-6.5 |
| Melting Point | 1640 (dec) |
| Density | 4.26 |
| Molecular Weight | mol. wt. = 79.9 |
| Refractive Index | 2.5-2.7 |
|}== Hazards and Safety ==
Nontoxic. Noncombustible.
No significant hazards.
LINK: International Chemical Safety Card
Additional Information
° M.Laver, "Titanium Dioxide Whites", Artists Pigments, Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997. ° Walter C. McCrone, "Polarized Light Microscopy in Conservation: A Personal Perspective" JAIC 33(2):101-14, 1994. (contains a table of dates on the history of titanium white as a pigment)
Comparisons
Properties of Common Abrasives
Natural and Simulated Diamonds
Characteristics of Common White Pigments
Sources Checked for Data in Record
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments"
- Artists' Pigments: A Handbook of their History and Characteristics, Elisabeth West FitzHugh, Oxford University Press, Oxford, Vol. 3, 1997 Comment: M.Laver, "Titanium Dioxide Whites" -In Norway, production of TiO2 patented in 1913, and pigment production in November 1918 (Titan Co. In U.S., patent applications filed in 1912 and production of a composite pigment began in 1916.
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 Comment: Pigment available in 1918
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9612
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Hoechst Celanese Corporation, Dictionary of Fiber & Textile Technology (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979 Comment: density=4.2-4.3, hardness=6.0-6.5
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- M. de Keijzer, 'A survey of red and yellow modern synthetic organic artists pigments discovered in the 20th century and used in oil colors', ICOM Preprints Lyons, France, Getty Conservation Institute, Los Angeles, p. 369, 1999
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Titanium_dioxide (Accessed Sept. 17, 2005)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 Comment: Artists pigments sold in mid 1920s
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- External source or communication Comment: Submission by N. Marco 8/25/05.
- Website address 1 Comment: Pigments Through the Ages -http://webexhibits.org/pigments/indiv/overview/tiwhite.html