Turmeric (Curcuma longa) LC
Description
Turmeric is considered "the most popular yellow colorant in the world" [1]. Turmeric is a perennial herb, which can grow up to 1 to 1.5m tall. The turmeric rhizomes have a bright orange-yellow.Turmeric is thought to be a native of India and nowadays cultivated all over southeast Asia and as far as the islands of the Pacific. Turmeric can be cultivated in most areas of the tropics and subtropics.
Turmeric rhizomes, fresh or dried, were grounded to powder to be used as dyes.
Historical Importance
As Tumeric is extensively used in ceremonies in India.Since the Vedic period (100-500 BC), and still today, turmeric has been part of Hindu civilization [2]. It is mentioned in Javanese and Balinese documents written before the mid-10th century AD.
Turmeric is used to dye the robes of Burmese Buddhist monks, on the bark cloth of Polynesia and Micronesia and in the faffia ikats of the Sakalva people of northwest Madagascar.
Research on textiles from China during the Ming and Qing dynasties has reported on usage of turmeric as dyeing resources[3, 4].
Examples:
Summary of results
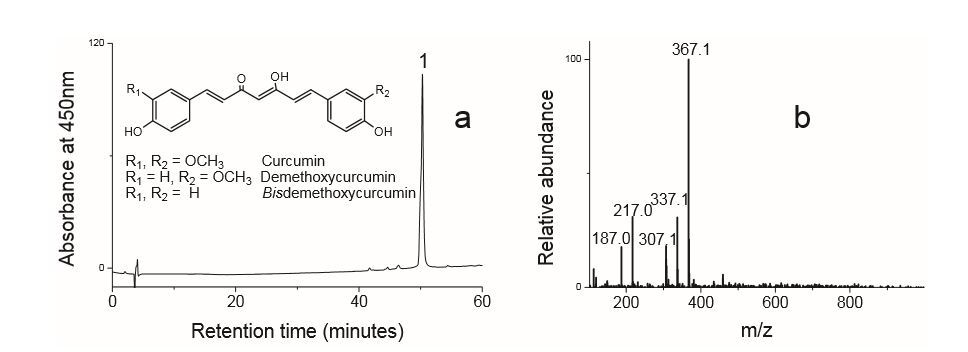
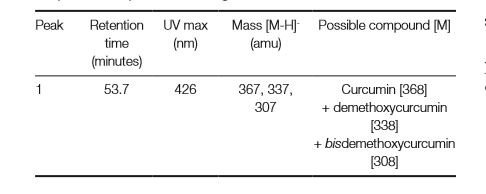
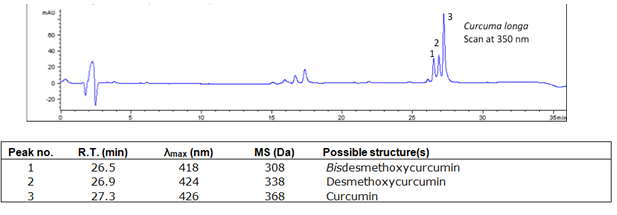
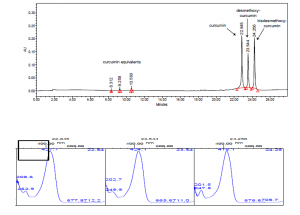
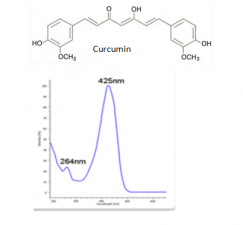
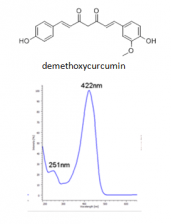
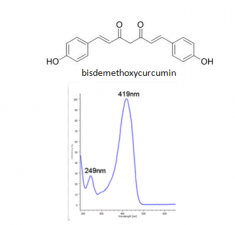
The major components of turmeric are three curcuminoids: curcumin, demethoxycurcumin and bisdemethoxycurcumin, as shown in the chromatogram. Curcumin is the major compounds. However, the contents of the minor compounds are important as they might depend mainly on the cultivar. The yellow Madras turmeric contans no more than 3.5% curcuminoids while the more orange Alleppy turmeric has 4.5-6%. [5]
Chromatograms
HPLC-DAD-MS extractants of dyes from a Qing Dynasty historical sample using a C4 column. HPLC-DAD-MS analysis was performed with an Agilent 1100 liquid chromatography system consisting of an automatic injector, a gradient pump, a HP series 1100 DAD, and an Agilent series 1100 VL on-line atmospheric pressure ionization electrospray ionization mass spectrometer. Separations were done on a Vydac 214TP52 analytical column (2.1 mm diameterX250 mm; 5-ím particle size). The column was eluted at a flow rate of 0.2 mL/min with a tertiary gradient of water (A),acetonitrile (B), and 1% (v/v) aqueous formic acid (C) with the following elution program: 0 min, 90% A, 5% B, 5% C; 0-55 min, a linear gradient to 35% A, 60% B, 5% C; 55-60 min, a linear gradient elution to 15% A, 80% B, 5% C; 60-62 min, isocratic elution at 15% A, 80% B, 5% C; 62-70 min gradient elution to 90% A, 5% B, 5% C; and reequilibration with the latter solvent for 15 min. The mass spectrometer was run both in the negative and positive ion mode.
HPLC-DAD-MS data using a C18 column.
Identified compounds
| Compound | RT (min.) | MW | UV/vis | Other | |
|---|---|---|---|---|---|
| curcumin | 53.7 | 368 | 278,352 | Comments here | |
| demethoxycurcumin | 53.7 | 338 | 280,395,370 | ||
| bisdemethoxycurcumin | 53.7 | 308 | 280,395,368 |
References
[1] Cardon, Dominique. "Natural dyes, sources, Traditions, Technology and Science" 318-322 (2007).
[2] Cardon, Dominique. "Natural dyes, sources, Traditions, Technology and Science" 169 (2007).
[3] Zhang, X., Corrigan, K., MacLaren, B., , M., and , R. A., Characterization of Yellow Dyes in Nineteenth Century Chinese Textiles. Studies in Conservation 52, 211-220 (2007).
[4] Han J. The Historical and chemical investigation of dyes in high status Chinese costume and textiles of the Ming and Qing Dynasties (1368-1911) PhD thesis, University of Glasgow February 2016.
[5] Cardon, Dominique. "Natural dyes, sources, Traditions, Technology and Science" 169 (2007).