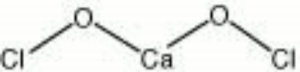
Calcium hypochlorite
Description
A white, crystalline solid. Calcium hypochlorite is a strong oxidizing bleach. It has been used in weak concentrations (0.5%) to bleach textiles and Paper although it may leave traces or residual chloride. It is also used as a Bactericide, Fungicide, and Disinfectant.
Synonyms and Related Terms
calcium hypochloride; bleaching powder; chloride of lime; calcium oxychloride; calcium salt of hypochlorous acid; Losantin; chlorinated lime
Hazards and Safety
Toxic chlorine gas may form when mixed with acids or ammonia.
Fire risk in contact with organic materials.
Severely corrosive to skin tissue on contact, inhalation, or ingestion.
The presence of trace heavy metals or their salts can accelerate oxidation.
Flinn Scientific: SDS
Physical and Chemical Properties
Decomposes in water and ethanol.
| Composition | Ca(OCl)2 |
|---|---|
| CAS | 7778-54-3 |
| Density | 2.35 |
| Molecular Weight | mol. wt. = 142.98 |
Resources and Citations
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Thomas Gregory, The Condensed Chemical Dictionary, Reinhold Publishing, New York, 3rd ed., 1942
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1648