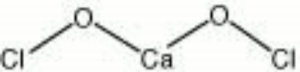
Calcium hypochlorite
Jump to navigation
Jump to search
Description
A white, crystalline solid. Calcium hypochlorite is a strong oxidizing bleach. It has been used in weak concentrations (0.5%) to bleach textiles and Paper although it may leave traces or residual chloride. It is also used as a Bactericide, Fungicide, and Disinfectant.
Synonyms and Related Terms
calcium hypochloride; bleaching powder; chloride of lime; calcium oxychloride; calcium salt of hypochlorous acid; Losantin; chlorinated lime
Risks
- Toxic chlorine gas may form when mixed with acids or ammonia.
- Fire risk in contact with organic materials.
- Severely corrosive to skin tissue on contact, inhalation, or ingestion.
- The presence of trace heavy metals or their salts can accelerate oxidation.
- Flinn Scientific: SDS
Physical and Chemical Properties
Decomposes in water and ethanol.
| Composition | Ca(OCl)2 |
|---|---|
| CAS | 7778-54-3 |
| Density | 2.35 g/ml |
| Molecular Weight | mol. wt. = 142.98 |
Resources and Citations
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Thomas Gregory, The Condensed Chemical Dictionary, Reinhold Publishing, New York, 3rd ed., 1942
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1648