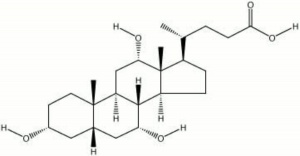
Cholic acid
Jump to navigation
Jump to search
Description
A bitter-tasting bile acid. Cholic acid is obtained naturally from ox bile extracts and commercially from Cholesterol. It is used as a natural sequestrant and detergent. Bile acid can neutralize PH and solubilize many inorganic salts. The sodium salt of cholic acid is the primary component in Oxgall.
Synonyms and Related Terms
bile acid; ox gall; ox bile extract
Risks
- Hygroscopic.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in acetic acid, acetone and ethanol. Slightly soluble in chloroform. Insoluble in water and benzene.
| Composition | C23H49O3COOH |
|---|---|
| CAS | 81-25-4 |
| Melting Point | 198-199 C |
| Molecular Weight | mol. wt. = 408.57 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Richard C. Wolbers, Nanette T. Sterman, Chris Stavroudis, Notes for Workshop on New Methods in the Cleaning of Paintings, J.Paul Getty Trust, Los Angeles, 1990
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 2258
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998