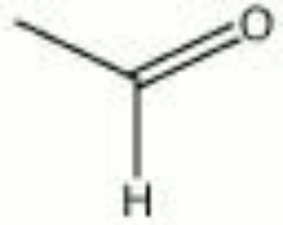
Acetaldehyde
Description
A colorless, volatile liquid with a characteristic fruity odor. Acetaldehyde reacts as a reducing agent when mixed with Tollen's reagent (ammonia - Silver nitrate) to form metallic silver on mirrors. It is used in the manufacture of paraldehyde, perfumes, flavors, aniline dyes, plastics, synthetic rubber, explosives. Acetaldehyde is also used to harden gelatin fibers and as a preservative and flavoring agent in foods and beverages.
Synonyms and Related Terms
ethanal (IUPAC); acetic aldehyde; aldehyde; ethyanal; ethyl aldehyde
Risks
Flammable, explosion risk, suspected carcinogen. Irritating to skin, eye and throat with the potential to cause burns and dermatitis.
ThermoFisher: MSDS
Other Properties
Miscible with water, ethanol, ether, benzene, gasoline, solvent naphtha, toluene, xylene, turpentine, acetone.
| Composition | CH2CHO |
|---|---|
| CAS | 75-07-0 |
| Melting Point | -123.5 |
| Density | 0.788 |
| Molecular Weight | mol. wt. = 44.05 |
| Refractive Index | 1.379 |
| Boiling Point | 21 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- ASTM, Standard Terminology Relating to Conditioning, Annual Book of ASTM Standards, Section 6, Paints, Related Coatings and Aromatics, ASTM, E41, 23-24, Sep-92
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.379