Difference between revisions of "Erythrosine"
(username removed) |
|||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A brown powder that forms a cherry red synthetic dye solution with water. First discovered in 1876 by Kussamaul, erythrosine is an iodinate derivative of [ | + | A brown powder that forms a cherry red synthetic dye solution with water. First discovered in 1876 by Kussamaul, erythrosine is an iodinate derivative of [[fluorescein]]. It is used as a direct dye on [[wool]] and [[silk]]. Erythrosine is also used in inks, lacquers, cosmetics, and as a lake pigment. It is not colorfast in [[solar radiation|sunlight]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Revision as of 09:49, 15 January 2014
Description
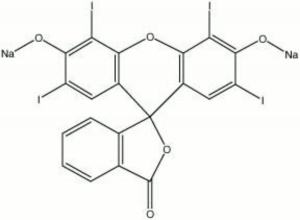
A brown powder that forms a cherry red synthetic dye solution with water. First discovered in 1876 by Kussamaul, erythrosine is an iodinate derivative of Fluorescein. It is used as a direct dye on Wool and Silk. Erythrosine is also used in inks, lacquers, cosmetics, and as a lake pigment. It is not colorfast in sunlight.
Synonyms and Related Terms
erythrosine B; erythrosine BS; Acid Red 51; CI 45430; FD&C Red No.3; Food Red 14; Pigment Red 172 (aluminum salt); Solvent Red 140; érythrosine (Fr.); eritrosina (Esp., Port.); sodium iodeosin; erythrosin
Other Properties
Soluble in water, ethanol. An aqueous solution of erythrosine will form a yellow-brown precipitate when drops of HCl are added and a red precipitate when drops of NaOH solution are added.
Maximum absorption wavelength = 524 nm.
The fluorescence of erythrosine changes with pH. It is colorless at pH 4.0 changing to a fluorescescent yellow-green at pH 4.5
| Composition | C20H6I4Na2O5 |
|---|---|
| CAS | 16423-68-0 |
| Molecular Weight | mol. wt. = 879.86 |
Hazards and Safety
Toxicity and carcinogenicity are being studied.
Mallinckrodt Baker: MSDS
Additional Information
M.Ballard (ed.), Important Early Synthetic Dyes. Chemistry, Constitution, Date, Properties. Conservation Analytical Laboratory, Smithsonian Institution, 1991.
Authority
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 3734
- External source or communication Comment: T.Schafer, C. Norton, V.Blyth-Hill, "The Efficacy of Using Boards Containing Zeolites in Passepartout for Works of Art on Paper, WAAC Newsletter, 22(1):14, 2000. -erythrosine fades rapidly
- Colour Index International online at www.colour-index.org Comment: discoverer = Kussmaul; CAS=16423-68-0, 12227-78-0, 15905-32-5
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: The fluorescence of erythrosine changes with pH. It is colorless at pH 4.0 changing to a fluorescescent yellow-green at pH 4.5
- Sigma Dyes, Stains and Natural Pigments, Infrared Library, Nicolet, 1991-1995 Comment: OMNIC for Erythrosine B: formula= C20H8I4O5, CAS= 15905-32-5 and for sodium salt of Erythrosine B: formula=C20H6I4Na2O5, CAS= 568-63-8