Difference between revisions of "Ethylene glycol monomethyl ether acetate"
Jump to navigation
Jump to search
(username removed) |
|||
| (3 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A clear liquid used as a solvent. Ethylene glycol monomethyl ether acetate is commonly called by its trademark name of methyl Cellosolve® acetate. It is a powerful solvent that dissolves most [ | + | A clear liquid used as a solvent. Ethylene glycol monomethyl ether acetate is commonly called by its trademark name of methyl Cellosolve® acetate. It is a powerful solvent that dissolves most [[natural resin|natural resins]] and [[synthetic resin|synthetic resins]]; it is used to make quick drying [[varnish|varnishes]], [[enamel, organic|enamels]], [[ink|inks]], stains and nail polishes. Methyl Cellosolve® acetate is also used to print alcohol soluble [[dye|dyes]] on [[textile|textiles]] and to make photographic film. |
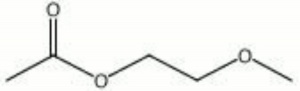
| − | + | [[[SliderGallery rightalign|ethylene glycol monomethyl ether acetate.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
methyl Cellosolve® acetate [Union Carbide]; 2-methoxyethyl acetate; 2-methoxy ethanol acetate | methyl Cellosolve® acetate [Union Carbide]; 2-methoxyethyl acetate; 2-methoxy ethanol acetate | ||
| − | + | == Risks == | |
| − | |||
| − | == | ||
| + | * Highly toxic by ingestion, inhalation and skin absorption. | ||
| + | * Potential carcinogen. | ||
| + | * Combustible. Moderate fire risk. | ||
| + | * PureChems: [https://www.pure-chemical.com/msds/Ethylene%20Glycol%20Mono%20ethyl%20Ether%20Acetate.pdf SDS] | ||
| + | == Physical and Chemical Properties == | ||
Miscible with ethanol, water, most organic solvents and oils. | Miscible with ethanol, water, most organic solvents and oils. | ||
| Line 22: | Line 25: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -65.1 | + | | -65.1 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.0067 | + | | 1.0067 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 34: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 145 | + | | 145 C |
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | ||
Latest revision as of 15:48, 5 August 2022
Description
A clear liquid used as a solvent. Ethylene glycol monomethyl ether acetate is commonly called by its trademark name of methyl Cellosolve® acetate. It is a powerful solvent that dissolves most natural resins and synthetic resins; it is used to make quick drying varnishes, enamels, inks, stains and nail polishes. Methyl Cellosolve® acetate is also used to print alcohol soluble dyes on textiles and to make photographic film.
Synonyms and Related Terms
methyl Cellosolve® acetate [Union Carbide]; 2-methoxyethyl acetate; 2-methoxy ethanol acetate
Risks
- Highly toxic by ingestion, inhalation and skin absorption.
- Potential carcinogen.
- Combustible. Moderate fire risk.
- PureChems: SDS
Physical and Chemical Properties
Miscible with ethanol, water, most organic solvents and oils.
| Composition | C5H10O3 |
|---|---|
| CAS | 110-49-6 |
| Melting Point | -65.1 C |
| Density | 1.0067 g/ml |
| Molecular Weight | mol. wt. = 118.1 |
| Boiling Point | 145 C |
Resources and Citations
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979